Factually inaccurate: Contrary to what the claim suggested, the amount of aluminum in childhood vaccines is below the safe limit for injectable biological products specified in the Code of Federal Regulations. An analysis of the pharmacokinetics of aluminum showed that the amount of aluminum from vaccines that enters the blood of babies isn’t dangerous.

FULL CLAIM: Per FDA guidelines, babies are too small to safely handle the amount of aluminum in childhood vaccines; “Per FDA guidelines, the max amount of aluminum anyone can safely handle is 4 micrograms per kilo. Most shots are 225 mcg to 850 mcg. So my kids would need to weigh a minimum of around 123 pounds to safely vaccinate”
REVIEW
Opponents to vaccination commonly point to the presence of aluminum in some vaccines as supporting evidence for their toxicity. Health Feedback explained that these claims are unsubstantiated in previous reviews.
Nevertheless, these claims persist. Take this Instagram post from February 2024 for instance, which argued that children receiving childhood vaccines would be exposed to amounts of aluminum exceeding the safe limit set by the U.S. Food and Drug Administration (FDA).
According to the post, most vaccines contain “225 micrograms to 850 micrograms” of aluminum, while “per FDA guidelines, the max amount of aluminum anyone can safely handle is 4 micrograms per kilo”. This means a child would need to weigh at least 56 kilograms to be able to safely receive a shot with 225 micrograms of aluminum (225 micrograms divided by four), and an unrealistic 212 kilograms to safely receive a shot with 850 micrograms of aluminum.
However, the reasoning behind this claim is flawed because its frame of reference for the safe limit of aluminum for children is incorrect. The claim that childhood vaccines contain unsafe levels of aluminum is also contradicted by published studies, as we will explain below.
For infants, diet is the greatest source of aluminum, not vaccines
Aluminum is the most abundant metal on earth. We’re constantly exposed to it through our water and our food, especially as a contaminant from manufacturing and packaging processes of industrial food[1]. The U.S. Agency for Toxic Substances and Disease Registry, part of the U.S. Department of Health and Human Services, published a report in 2008 estimating that adults in the U.S. eat seven to nine milligrams of aluminum per day.
Aluminum salts are also used as adjuvants in some vaccines. Adjuvants are molecules that boost the reaction of the immune system to a vaccine. They help ensure that a vaccine will stimulate the immune system in such a way that it will generate a lasting immune memory against the pathogen.
According to the Agency for Toxic Substances and Disease Registry, the concentration of aluminum ranges from 0.0092 to 0.049 milligrams per liter (mg/L) in breast milk, 0.46 to 0.93 mg/L in soy-based formula, and 0.058 to 0.15 mg/L in milk-based formula.
In addition, the Children’s Hospital of Philadelphia calculated that children absorb between seven and 117 milligrams of aluminum through their diet during their first six months of life and receive only 4.4 milligrams through childhood vaccines.
Therefore, babies receive most of the aluminum they’re exposed to through their diet, not vaccines.
The safe limit for aluminum in vaccines is higher than claimed
The claim didn’t provide its source for the cited limit on aluminum (four micrograms per kilogram of body weight). However, we found one FDA document providing guidance for labeling aluminum content for products related to parenteral nutrition (PN)—receiving nutrition intravenously—that may be the source for this claim. It states:
“FDA recommends that the total aluminum exposure (TAE) from PN uniformly should not exceed 5 mcg/kg/day to protect the safety of all patients.”
However, this limit doesn’t apply to vaccination. The group of people who need to receive parenteral nutrition isn’t representative of the general population. This form of feeding is mostly used for people whose kidneys aren’t working properly and for babies who were born prematurely and whose kidneys aren’t functional yet, according to the same FDA document.
Kidneys are also responsible for eliminating the aluminum that circulates in the blood. Indeed, 99% of the ingested aluminum that passes into the blood is excreted by the kidneys. Only 0.5% of the aluminum remains in the bloodstream 24 hours later[1].
The FDA explained:
“Research indicates that patients with renal impairment, including preterm neonates, who receive parenteral levels of aluminum at greater than 4 to 5 micrograms/kilogram/day (mcg/kg/day) accumulate aluminum at levels associated with central nervous system and bone toxicity”
And:
“Because patients with renal impairment, including all preterm neonates, comprise a major portion of those requiring [parenteral nutrition] support, FDA recommends that the total aluminum exposure (TAE) from PN uniformly should not exceed 5 mcg/kg/day to protect the safety of all patients”, the FDA said.
Thus, people who require long-term parenteral nutrition are often those whose bodies aren’t able to properly eliminate aluminum—hence the limit of five micrograms per kilogram of body weight per day established by the FDA.
By contrast, healthy children receiving childhood vaccines have functional kidneys, which are able to quickly eliminate aluminum. Therefore, this limit on aluminum content may not be relevant to childhood vaccination.
In fact, the Code of Federal Regulations (CFR) indicates another limit for the amount of aluminum in injectable biologics, including vaccines. It states that the amount of aluminum shall not exceed between 0.85 and 1.25 milligrams per dose, depending on the technique used to measure the amount of aluminum.
And as we can see in this list of vaccine ingredients provided by the Children’s Hospital of Philadelphia, quantities of aluminum in recommended childhood vaccines don’t exceed that limit.
The amount of aluminum from vaccines doesn’t exceed the minimal risk level, study shows
Although we know that aluminum content in vaccines is within the safe limit established by the CFR, as explained above, it’s important to consider one limitation. Indeed, the CFR limit is per dose, and thus doesn’t translate easily into an amount of aluminum per child body weight.
This nuance is important because a dose of vaccine administered to a baby compared to a dose given to a full-grown adult would result in a different concentration of aluminum in the body due to size differences. Therefore, it is necessary to assess whether administering those vaccines to young infants would be indeed safe.
The U.S. Centers for Disease Control and Prevention (CDC) referenced a research by Mitkus and colleagues that set out to answer that question by calculating the aluminum burden in the babies’ bodies[2]. To do so, they took several factors into consideration.
First, they used the minimal risk level (MRL) for aluminum of one milligram per kilogram of body weight per day, when ingested, as established by the Agency for Toxic Substances and Disease Registry.
Contrary to the CFR limit, the MRL is determined by body weight, so it can be adapted to the lower and continuously increasing weight of infants. This MRL is also more relevant to vaccines than the FDA’s limit on aluminum for parenteral nutrition, because it can be applied to the whole population—not only those suffering from renal dysfunction.
Second, Mitkus et al. converted the safe limit of ingested aluminum to a safe limit of aluminum circulating in the blood. They did this by considering that only a small fraction of the ingested aluminum crosses the gastrointestinal tract into the bloodstream.
Finally, they took in consideration the fact that aluminum is continuously being eliminated from the blood through the kidneys and that vaccination is usually done through intramuscular injection, not intravenous injection.
While all the aluminum from an intravenous injection would enter the blood, meaning that aluminum levels in the bloodstream would quickly rise after the injection, most of the aluminum from an intramuscular injection would initially remain in the muscle. This aluminum would gradually enter the bloodstream over time, meaning that systemic exposure to aluminum is smaller compared to that from intravenous injection[2].
After accounting for all these variables, Mitkus et al. came to the conclusion that the amount of aluminum administered through a baby’s diet and childhood vaccines didn’t exceed the minimal risk level (Figure 1). In other words, available results indicated that the amount of aluminum in the blood following vaccination is safe for babies.
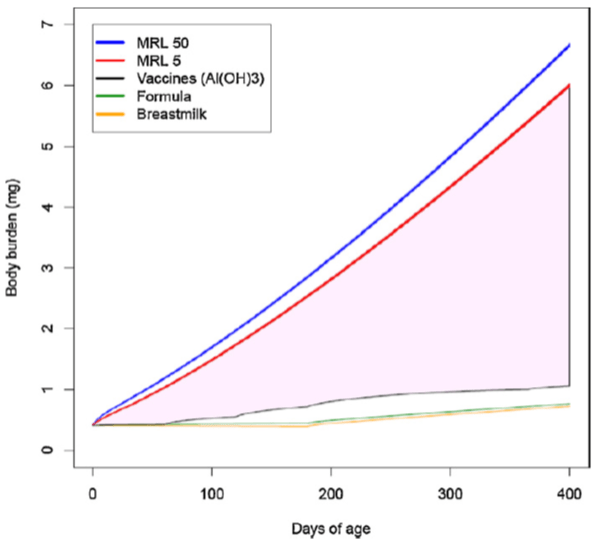
Figure 1 – Body burden of aluminum from diet and vaccination. The red and blue lines represent the minimal risk level (MRL) of aluminum in the blood of babies. This level increases with the baby’s age as it gets bigger. The black line represents the amount of aluminum in the blood originating from vaccination according to the childhood vaccination schedule. Source: Mitkus et al.[2].
In summary, the amount of aluminum contained in some childhood vaccines doesn’t exceed the U.S. Federal limits. While some may worry that this amount is higher than the FDA guidelines for parenteral nutrition products, this overlooks the fact that parenteral nutrition in infants primarily concerns babies who are born prematurely and don’t have functional kidneys yet that can eliminate aluminum. This is not the case for the majority of babies, whose bodies can effectively filter out aluminum. Available evidence indicates that the amount of aluminum from vaccines in babies’s blood doesn’t exceed the minimal risk level.
REFERENCES
- 1 – Corkins et al. (2019) Aluminum Effects in Infants and Children. Pediatrics.
- 2 – Mitkus et al. (2011) Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine.


