Unsupported: Scientific evidence does not support the claim that vaccines are associated with neurological damage or autism. The study cited to support this claim is deeply flawed.

FULL CLAIM: Aluminum in vaccines causes neurological damage and autism
SUMMARY
Several Facebook posts containing screenshots of Google search results purporting to show evidence linking aluminum in vaccines with neurological damage and autism were published in early March 2020. An article published by Collective Evolution in mid-February reported a similar claim made by Christopher Exley, professor of bioinorganic chemistry at Keele University, based on the findings of his study[1].
Scientists who specialize in neuroscience explained to Health Feedback that the claim was not supported by the bulk of scientific evidence.
Abraham Al-Ahmad, assistant professor at Texas Tech University Health Sciences Center, explained that: “Aluminum is present in vaccines as microparticles, with a particle size ranging from 1 to 10 micrometers[2]. Such particles are too big to cross cellular barriers, especially the blood-brain barrier (BBB). To put this into perspective, tight junctions at the BBB have a pore size of about 4 angstroms (0.0004 micrometers).” Studies have also shown that aluminum release into the bloodstream from the site of injection is extremely slow—it has been estimated that more than 80% of aluminum remains at the injection site 28 days post-injection[3-5]. [See below for Al-Ahmad’s full comment.]
The amount of aluminum that an individual might receive from a vaccine is minuscule compared to the amount that infants receive in their diet. As explained by the Children’s Hospital of Philadelphia:
“The aluminum contained in vaccines is similar to that found in a liter (about 1 quart or 32 fluid ounces) of infant formula. While infants receive about 4.4 milligrams* of aluminum in the first six months of life from vaccines, they receive more than that in their diet. Breast-fed infants ingest about 7 milligrams, formula-fed infants ingest about 38 milligrams, and infants who are fed soy formula ingest almost 117 milligrams of aluminum during the first six months of life.”
Reviewers also criticized the study led by Exley, which was used as the basis for the Collective Evolution article and supposedly showed “high amounts of aluminum in the brain tissue of people with autism”[1]. However, reviewers pointed out that the study never compared these aluminum levels with those of brain tissues from people without autism (control group). Therefore, it is impossible to determine whether the reported aluminum levels were abnormally high, or if they were similar to people without autism, invalidating the study’s conclusion.
As reported in Health Feedback’s earlier reviews, vaccine ingredients are safe and vaccines are not associated with autism.
After our evaluation was published, the Daily Mail corrected its article to clarify that Exley’s claim is unsupported and removed all false/misleading content from the article (archive of original article). See the archive of the corrected article.
SCIENTISTS’ FEEDBACK
Abraham Al-Ahmad, Assistant Professor, Texas Tech University Health Sciences Center:
This statement is of very low scientific credibility, inaccurate, and involves flawed reasoning.
A common tactic used by anti-vaccine activists is to use the existing literature that documents the neurotoxicity of aluminum (most of the time given at important doses, using chemical formulation of aluminum salts different from the adjuvants used in vaccines) and directly apply these findings to claim that aluminum in vaccines is toxic.
My comments are divided into two sections:
- Chemical properties of aluminum adjuvants present in vaccines
Aluminum adjuvants in vaccines exist in two major species: aluminum hydroxide (AH) and aluminum phosphate (AP). These are aluminum salts that have a very slow dissociation profile, especially at biological pH (7.4), therefore these salts have one of the lowest solubility constant rates (Ks). The dissolution of aluminum results in the formation of different species, however the biologically active aluminum takes the form of free Al3+ ions.
Aluminum is present in vaccines as microparticles, with a particle size ranging from 1-10 micrometers (µm)[2]. Such particles are too big to cross cellular barriers, especially the blood-brain barrier (BBB). To put this into perspective, tight junctions at the BBB have a pore size of ~4 angstroms [0.4 nanometers (nm) = 0.0004 µm].
Vaccines are commonly injected via the intramuscular (IM) route, which occurs in a tissue with a poor perfusion rate (similar to skin and fat tissue). If we use this as a reference, we can see that skin and muscles are considered poorly perfused tissues with a flow rate < 5 mL/min/100g tissue, compared to the brain (55 mL/min/100g tissue) or heart (84 mL/min/100g). The release of aluminum into the bloodstream is a slow process, both in terms of reaching a peak concentration (Cmax) occurring around 10 hours post-injection[3] and a very slow release. It is estimated that over 80% of the aluminum injected at the injection site still remains after 28 days post-injection[3-5].
In the most recent study, Weisser and colleagues failed to distinguish an increased aluminum blood concentration that was statistically higher than the background concentration reported in the vehicle-treated (saline) control group[5]. Such injection profiles match the dose administered in the 2-month visit in the CDC immunization schedule (850 µg of aluminum).
Circulating Al3+ in the bloodstream is mostly bound to transferrin or to citrate. A study by Yokel and colleagues investigated the pharmacokinetic profile and brain uptake of 26Al (a stable radioactive form of Al) following intravenous bolus injection[6]. In this study, Yokel and colleagues reported an uptake of ~0.001-0.005% of the injected aluminum per gram of rat brain (the weight of the average rat brain is about 2 g).
Taken together, the evidence shows that the amount of aluminum from vaccines is very unlikely to increase the circulating aluminum levels in plasma so as to be a concern, while the amount of aluminum crossing the BBB is too low to represent a concern. Therefore, the claim that aluminum from vaccines is responsible for neurotoxicity (and therefore neurological diseases) is near-impossible.
- Critique of the scientific study by Mold et al.
The Collective Evolution article reported that a study published by a researcher from Keele University reported a high level of aluminum in autistic brains. This study was published by Mold and colleagues under the title of “Aluminium in brain tissue in autism” in the Journal of Trace Elements in Medicine and Biology in 2018[1]. The senior author in this publication is Christopher Exley, a scientist from Keele University. Dr. Exley is a long-time scientist who has been challenging the safety of aluminum adjuvants. According to his publication records, Exley considers that a high load of aluminum is responsible (or associated) with various neurological diseases including Alzheimer’s disease, autism, epilepsy, or multiple sclerosis.
There are several issues with this study that need to be highlighted:
a. The lack of a control group for brain samples:
The presence of a control group is the tenet of the scientific method since its establishment by Claude Bernard [in 1865]. The absence of a control group makes it impossible to determine whether any outcomes measured in this study are considered normal or pathological. Interestingly, Exley has published a study previously in which he had access to control brain levels[7].
b. The sample size:
Sample size (N) is an important parameter for statistical analysis, as it will dictate the power of analysis of a study, and eventually its meaningfulness when it comes to statistical significance. On average, we consider an N of 8-10 animals/group as the bare minimum for establishing a power of analysis. For clinical and epidemiological studies, you need at least an N of >50-100 to reach a decent power of analysis (rule of thumb: the more, the better). In this study, the sample size was 5, which is perhaps acceptable for a pilot study and for speculating on a hypothesis, but certainly not enough to make this bold statement about aluminum and autistic brains.
It is also important to note that the authors failed to classify such donors or provide information on the type of autism associated with the patient and standardized diagnosis (using the DSM-V classification). This is important, as autism is a spectrum of disorders, rather than a clean and distinctive condition.
c. The extreme variability in the techniques:
An important criterion for assessing the relevance of experimental data is the signal-to-noise ratio of a technique. This will usually take the form of an average and standard deviation. The higher the standard deviation is, the more noise (or variability) is present, and the less accurate the conclusion.
In this study, Exley and his team measured aluminum levels in three pieces of a brain sample taken at different regions (parietal, occipital, temporal, and frontal cortex regions). To reduce the noise (and increase measurement accuracy), you can perform technical replicates for the same biological sample. This is a good option to increase the accuracy of your results, but you cannot use technical replicates as biological replicates. This lack of clarity in the methodology is a concern, because we can observe an important variability between biological samples from the same patient, but also an important variability between samples from different patients.
At this point, we cannot distinguish if such variability is inherent to uneven distribution of aluminum within the same brain region, or sample contamination by external aluminum during the brain extraction and preservation (formalin-fixed brain), as aluminum is the third most-common element on Earth’s crust.
d. The absence of duplicate methodology to address the same outcome:
In science, it is important to show that you have obtained the same results or arrive at the same conclusion using two distinct methods. In this study, we are only given one qualitative method (immunofluorescence) and one quantitative method (graphite furnace atomic absorption spectrometry). The use of a single technique to address one outcome (e.g., aluminum content in tissue) can be prone to erroneous measurements and biased conclusions.
e. The questionable conclusions made by the authors:
In the Discussion, the authors stated the following: “However, the fact that we found aluminium in every sample of brain tissue, frozen or fixed, does suggest very strongly that individuals with a diagnosis of autism spectrum disorder (ASD) have extraordinarily high levels of aluminium in their brain tissue”. Yet, the absence of controls makes such a conclusion impossible. Worse, if we directly compare the values of aluminum reported in brain tissue from people with ASD to values reported in control brains (see Figure 1 below), we fail to see a statistically significant difference between the control group and the ASD group. Using his own publications, we can see Exley contradicting himself on that claim.
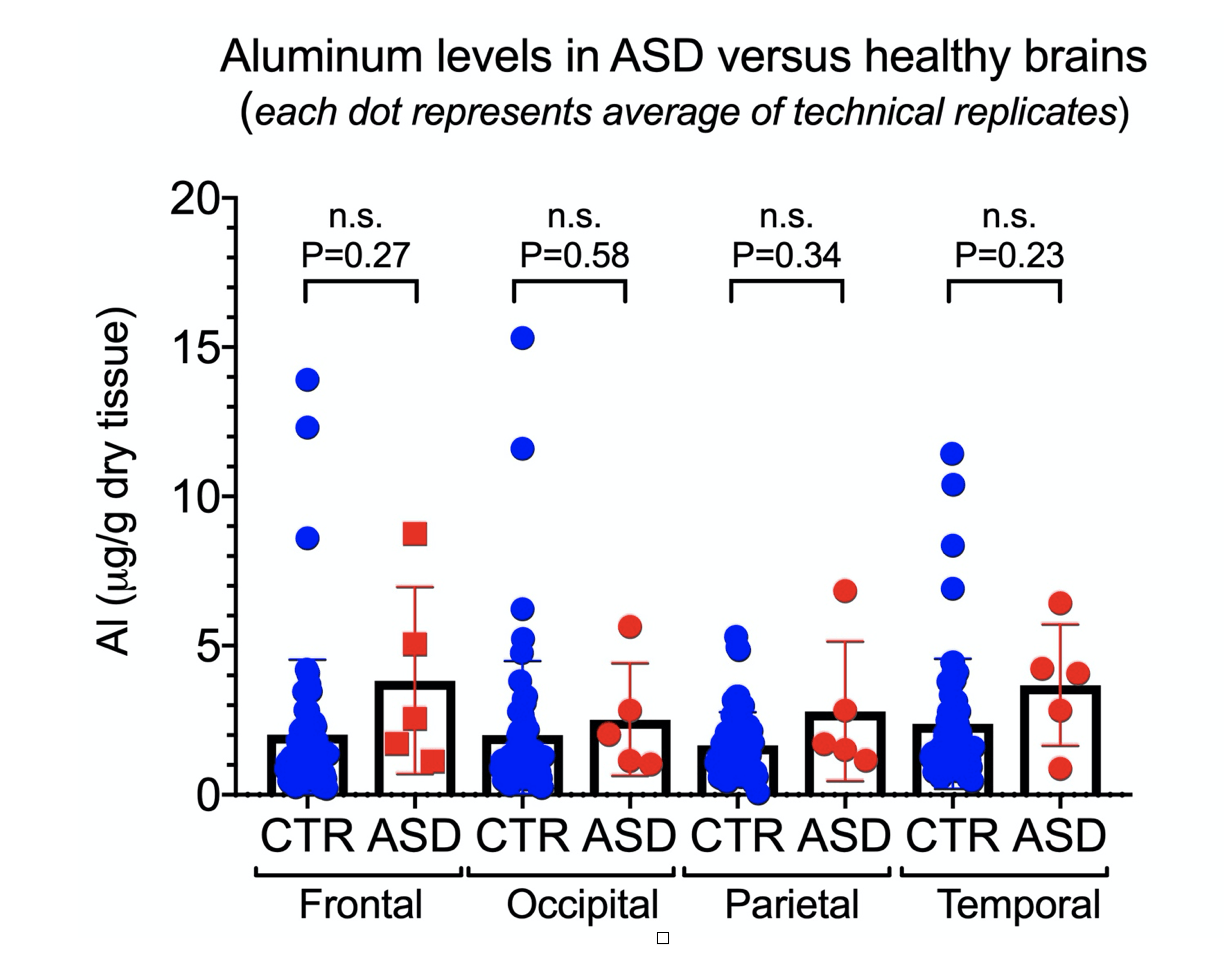
Figure 1. Comparison of aluminum levels between brain tissues of individuals with autism spectrum disorder (ASD)[1] and control (non-ASD) group[7] showing no significant difference between the two groups.
The second claim pushed by Exley is the observation of aluminum deposition in brain tissues (using Lumogallion staining), to the extent of precisely identifying the cell type in which such deposition occurs.
Exley claims to identify cells visually without the use of cell type-specific markers, as reported in the text as: “Aluminium-loaded mononuclear white blood cells, probably lymphocytes,” or “Aluminium was located in inflammatory cells associated with the vasculature”, or “Glial cells including microglia-like cells that showed positive aluminium fluorescence were often observed in brain tissue in the vicinity of aluminium-stained extracellular deposits”. Even a seasoned neuropathologist will find it highly difficult to identify a cell type without the proper staining techniques or the use of antibodies targeting specific cellular markers expressed by different cell types (e.g., GFAP for astrocytes, NeuN for neurons, CD3 for immune cells).
It is also important to note the lack of negative controls (tissue samples without lumogallion stain) to ensure such fluorescence is not autofluorescence due to lipids enriched in the brain (e.g., lipofuscin), as well as the absence of brain tissue from a control group to determine whether such features observed are specific to ASD brains.
Finally, the staining of the aluminum particles reported in Exley’s study looks a bit strange, mostly looking like blobs or stained debris (see Figure 2 below). A detailed analysis of their size indicates spherical structures of about 5 µm, almost perfect. Again, we know that aluminum microparticles are too big to cross the BBB, and the consistent size within panels makes us wonder what these structures are. This is likely bleed-through fluorescence as lipofuscin, which naturally occurs inside certain cells, can emit at the same emission spectra as lumogallion, and thus could have given spurious signals resembling that of lumogallion.
Avoiding this would require a very good microscope which only allows a specific range of excitation and emission wavelength to cross the sample and reflect the fluorescent light to the microscope. The microscope model used in the study seems to lack these capabilities. Differentiating potential fluorescence from lipofuscin from that of lumogallion can be also done by imaging unstained tissue (i.e., negative controls) and stained tissue from non-ASD individuals, using an exposure time in which you can barely get any fluorescence captured by the microscope’s camera. However, such negative controls are lacking in the paper.
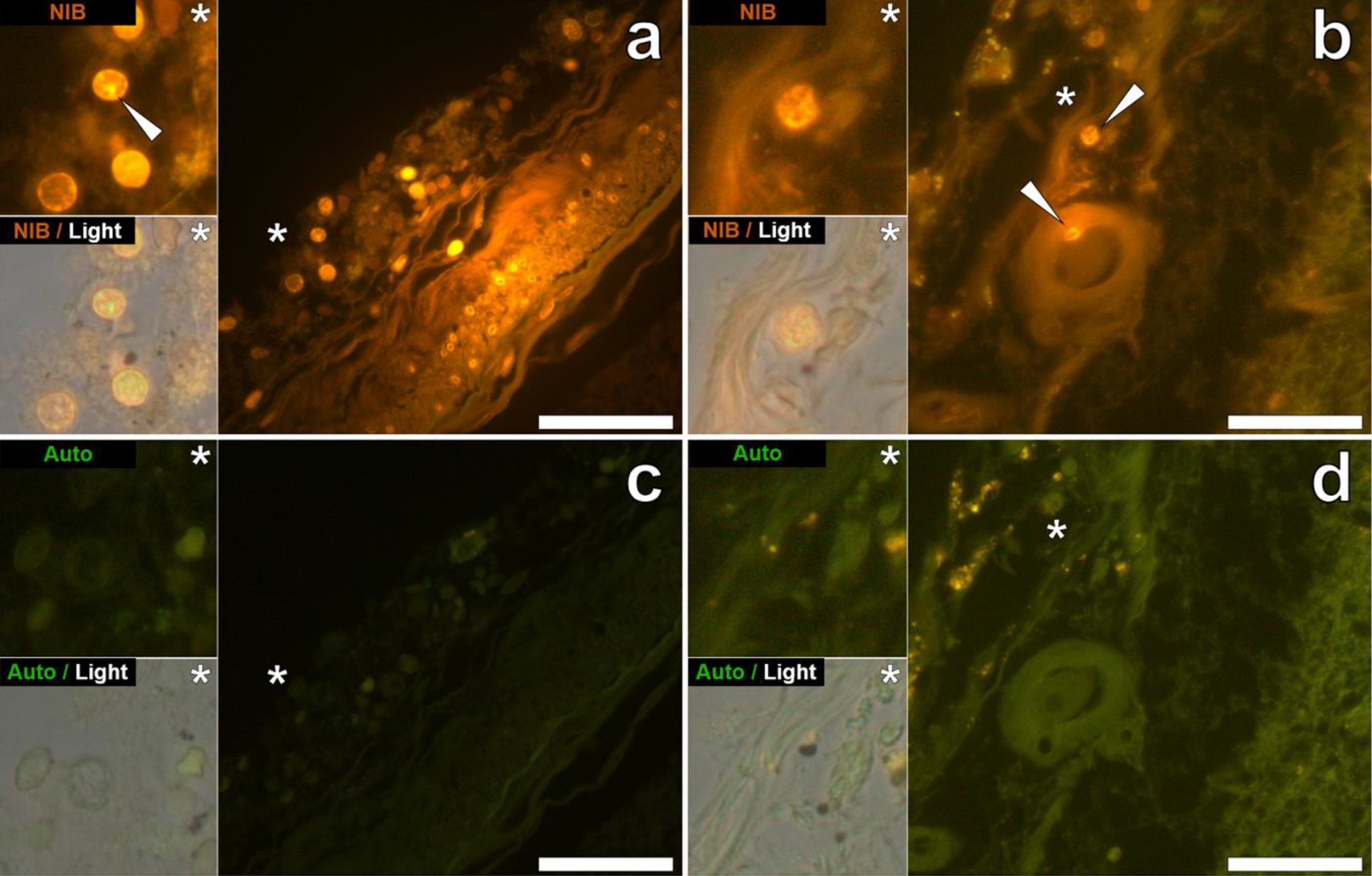
Figure 2. Lumogallion staining of perfectly round structures purported to be aluminum within cells in the hippocampus (a) and frontal lobe (b) as reported by Mold et al.[6] Unstained sections shown in panels c and d.
Another interesting point is that the presence of detergent (like Triton-X 1%) was enough to trigger a significant increase in the excitation and emission spectra. Given that white matter of the brain is myelin-rich and thus enriched in lipids, we cannot exclude a possible false positive signal coming from the staining (or even from the use of Triton-X to permeabilize the tissue), and this seems confirmed by the high background fluorescence level.
If Exley wants us to trust his pictures, I would recommend him to find a confocal microscope and let a seasoned pathologist do the staining with specific cell markers. This will allow a much more convincing conclusion than this inconclusive series of immunofluorescence pictures that show gigantic fluorescent blobs—which look more like artifacts than credible sub-cellular features—will allow.
* There are some troubling elements about Exley’s publication records that need to be highlighted.
Firstly, I am surprised that the paper by Mold et al. was accepted as-is without reviewers objecting to it. At the time of review and publication, Exley was a sitting member of the editorial board. That does not necessarily mean that Exley reviewed his own paper, but there is a possible conflict of interest that cannot be dismissed.
The peer review timeline is also concerning. This is what the paper says: “Received 26 October 2017; Received in revised form 21 November 2017; Accepted 23 November 2017”[1]. So it was received on Thursday October 26th, then reviewed, revised by the authors and resubmitted on Tuesday November 21st. That’s a very diligent review: just 2 weeks’ turnover, which would put the editor’s response to the authors sometime between Thursday November 9th and Monday November 13th) and Exley rapidly replying to the editor (about a week to revise and resubmit).
It is also important to report that Exley had submitted two corrections to two of his publications[8,9]. In both publications, Exley failed to disclose a conflict of interest as he received funding from the Children’s Medical Safety Research Institute (CMSRI), an anti-vaccine organization funding agency for which Exley is acting as a scientific advisor.
Furthermore, Exley co-authored a letter to the editor of the journal Toxicology in 2018 in response to a letter by other scientists expressing concerns towards a study led by Exley. The letter by Exley et al. was later retracted because it was considered “slanderous”.
More recently, Exley has been providing research support for the Spritzer water bottling company in promoting “silica-rich” water as a preventive and cure for Alzheimer’s disease, claiming the silicon-rich water will clear the brain of the aluminum overload or prevent aluminum deposition into the brain[10] (which he considers a primary causal agent for Alzheimer’s disease[11]). In turn, this company was sponsoring Exley’s research until recently, as shown in the disclaimer at the bottom of this archived webpage.
Marta Garcia-Miralles, Senior Research Fellow, Department of Physiology, National University of Singapore:
It is well known that aluminum is the most predominant metal in the earth’s crust (8.8%). The main sources of aluminum found in the human body come from inhaling it in the air (<3%), food consumption (<1%), skin penetration in adults with healthy skin (<0.01%)[12], and consumption of aluminum-containing medicinals. Lastly, aluminum salts have been incorporated into some vaccine formulations to enhance the immune response of vaccinated individuals.
There is no evidence to support the claim that aluminum adjuvants are neurotoxic. According to the U.S. Food and Drug Administration (FDA), aluminum adjuvant-containing vaccines have “a demonstrated safety profile of over six decades of use and have only uncommonly been associated with severe local reactions”. In the same line, the Global Advisory Committee on Vaccine Safety (GACVS) at the World Health Organization (WHO) reported no evidence of health risk from aluminum-containing vaccines. Other studies carried out by the FDA demonstrate that the total aluminum exposure received from the entire recommended series of childhood vaccines over the first year of life is extremely low and the risk is not significant[13,14]. Also, it is interesting to point that the amount of aluminum in the hepatitis B vaccine given at birth is 0.25 mg[13,14], and the total body burden of aluminum in healthy individuals is 30–50 mg according to the Agency for Toxic Substances & Disease Registry.
Some reports have shown evidence of neurotoxicity due to excessive accumulation of aluminum in a condition known as dialysis encephalopathy syndrome which could possibly be caused by aluminum intoxication. In 1976 it was demonstrated that the encephalitis observed in dialysis patients with renal failure was caused by excessive accumulation of aluminum in the brain that could not be excreted due to kidney failure[15]. Metal accumulation was due to aluminum intake from aluminum in dialysis water and antacid drug intake containing aluminum salt. Following kidney transplantation and excretory function restoration, the encephalopathy progressively regresses because of urinary excretion of aluminum stocked in the brain[12]. Therefore, the cause of encephalopathy is renal failure resulting in aluminum accumulation.
Some local effects have been reported in people following injection of aluminum-containing vaccines, especially macrophagic myofasciitis. Muscle biopsies at the injection site revealed the presence of aluminum salts as crystalline inclusions in macrophages, but without any significant myocytic lesion nor necrotic lesion. This is supported by an animal study which found that these are “not associated with abnormal clinical signs”[16].
The article published in Collective Evolution reported a scientist’s view that aluminum and aluminum adjuvants used in vaccines “almost certainly” play a role in autism. The author describes the study published by Christopher Exley in 2018 where he and his team reported high amounts of aluminum in the brain tissue measured in five donors diagnosed with autism spectrum disorders (ASD). Exley et al. reported that the content of aluminum for the whole brain ranged from 1.20±1.06 µg/g to 4.77±4.79 µg/g (by dry weight)[1].
However, in order to know whether these values are abnormally high, one would need to compare them with aluminum content in brain tissue from a control (i.e., non-ASD) group. These controls are missing in the original paper, therefore this statement is not supported by the data. In addition, the article does not mention that the authors of the study acknowledged a significant inter-tissue (sample replicates), inter-lobe, and inter-subject variability of the aluminum content. This statement calls into question the reproducibility of the method as well as the study’s reliability.
According to an interview given by Exley, he and his colleagues identified the majority of aluminum to be localized intracellularly in neurons and non-neuronal cell populations, and found evidence that cells in the lymph and the blood were carrying aluminum and passing into the brain. However, in the original study it is stated that they were only supplied with 3 serial sections of each tissue, so they were not able to do any staining for general morphology. This means that it was not always possible to determine which type of cell was showing aluminum fluorescence. Clearly, this statement contradicts Exley’s claim and calls into question again the reliability of the data. Once more, the author of the Collective Evolution article decided to omit this statement.
Finally, in the last part of the interview, Exley explained that his team showed how aluminum adjuvants can be taken up by the same cells observed in tissue from people with autism and at the injection sites, thus claiming a link between aluminum as an adjuvant for vaccines. Specifically, they hypothesized that this same aluminum was potentially carried by those cells across the blood-brain barrier into the brain tissue, where it could deposit and produce disease or a more severe and disabling form of autism. The article does not provide a reference to support such a statement. Also, as mentioned above, the claim that aluminum is carried by lymph and blood cells across the blood brain barrier is not supported by the data published in 2011 either.
Furthermore, the premise of this study is based on a 2011 report by Tomljenovic and Shaw, claiming aluminum in vaccines is associated with autism, that was considered “seriously flawed” by the Global Advisory Committee on Vaccine Safety (GACVS) at the World Health Organization (WHO) in July 2012. According to GACVS, the study is based on ecological comparison of aluminum content in vaccines and rates of autism spectrum disorders in several countries —such ecological studies cannot be used to assert a causal association since they do not link exposure to outcome in individuals. In addition, other concerns were found in this study that would limit the value of these studies for hypothesis generation.
The WHO also reported that the available epidemiological data conclusively show that there is no evidence of a causal association between measles, mumps, and rubella vaccines and ASD, since previous studies suggesting a causal link were found to be filled with methodological flaws. Also, there is no evidence to suggest that any other childhood vaccine may increase the risk of ASD. A review of the evidence for a potential association between aluminum adjuvants contained in inactivated vaccines and the risk of ASD clearly indicate that vaccines do not increase the risk of ASD.
In conclusion, the main claim of the article is based on a wrong premise and a poorly designed study.
James D. Cherry, Professor (Pediatrics), David Geffen School of Medicine, University of California Los Angeles:
[This comment comes from an evaluation of a related claim.]
There is aluminum salt present in killed vaccines, such as the present DTaP and Tdap vaccines, but it’s a very good adjuvant and it’s been in killed vaccines from the very beginning. The amount and the actual aluminum salt may vary with vaccines. The key thing is that too much aluminum would give you abscesses at the site of vaccination, but no present killed vaccine has excessive amounts. In live vaccines there’s no aluminum at all.
Vaccines are incredibly safe. The things that are blamed on vaccines —such as sudden infant death syndrome, have been proven to be false[17,18,19,20]. They are also blamed for the so-called “vaccine encephalopathy”, and that’s been proven in studies to be wrong[20,21,22,23,24,25].
The benefits arising from vaccines can be illustrated by the example of the measles vaccine, which on its own prevents approximately 500 deaths a year in the United States. All our present vaccines are incredibly effective and these claims about vaccine ingredients are wrong.
REFERENCES
- 1 – Mold et al. (2018) Aluminium in brain tissue in autism. Journal of Trace Elements in Medicine and Biology.
- 2 – Shardlow et al. (2016) From Stock Bottle to Vaccine: Elucidating the Particle Size Distributions of Aluminum Adjuvants Using Dynamic Light Scattering. Frontiers in Chemistry.
- 3 – Flarend et al. (1997) In vivo absorption of aluminium-containing vaccine adjuvants using 26Al. Vaccine.
- 4 – Weisser et al. (2019) Aluminium toxicokinetics after intramuscular, subcutaneous, and intravenous injection of Al citrate solution in rats. Archives of Toxicology.
- 5 – Weisser et al. (2019) Aluminium in plasma and tissues after intramuscular injection of adjuvanted human vaccines in rats. Archives of Toxicology.
- 6 – Yokel et al. (2001) Entry, half-life, and desferrioxamine-accelerated clearance of brain aluminum after a single (26)Al exposure. Toxicological Sciences.
- 7 – House et al. (2012) Aluminium, iron and copper in human brain tissues donated to the Medical Research Council’s Cognitive Function and Ageing Study. Metallomics.
- 8 – Exley et al. (2019) Correction to: Aluminium in human brain tissue: how much is too much? Journal of Biological Inorganic Chemistry.
- 9 – Mold et al. (2019) Correction to: Unequivocal imaging of aluminium in human cells and tissues by an improved method using morin. Histochemistry and Cell Biology.
- 10 – Davenward et al. (2013) Silicon-rich mineral water as a non-invasive test of the ‘aluminum hypothesis’ in Alzheimer’s disease. Journal of Alzheimer’s Disease.
- 11 – Exley C. (2017) Aluminum Should Now Be Considered a Primary Etiological Factor in Alzheimer’s Disease. Journal of Alzheimer’s Disease Reports.
- 12 – Goullé and Grangeot-Keros. (2020) Aluminum and vaccines: Current state of knowledge. Médicine et Maladies Infectieuses.
- 13 – Mitkus et al. (2011) Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine.
- 14 – Keith et al. (2002) Aluminum toxicokinetics regarding infant diet and vaccinations. Vaccine.
- 15 – Alfrey et al. (1976) The Dialysis Encephalopathy Syndrome — Possible Aluminum Intoxication. New England Journal of Medicine.
- 16 – Verdier et al. (2005) Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the Cynomolgus monkey. Vaccine.
- 17 – Hoffmann et al. (1987) Diphtheria-tetanus-pertussis immunization and sudden infant death: results of the National Institute of Child Health and Human Development Cooperative Epidemiological Study of Sudden Infant Death Syndrome risk factors. Pediatrics.
- 18 – Mitchell et al. (1995) Immunisation and the sudden infant death syndrome. Archives of Disease in Childhood.
- 19 – Vennemann et al. (2005) Do immunisations reduce the risk for SIDS? A meta-analysis. Vaccine.
- 20 – Müller-Nordhorn et al. (2015) Association between sudden infant death syndrome and diphtheria-tetanus-pertussis immunisation: an ecological study. BMC Pediatrics.
- 21 – Doja (2008) Genetics and the myth of vaccine encephalopathy. Journal of Paediatrics and Child Health.
- 22 – Berkovic et al. (2006) De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. The Lancet Neurology.
- 23 – McIntosh et al. (2010) Effects of vaccination on onset and outcome of Dravet syndrome: a retrospective study. The Lancet Neurology.
- 24 – Reyes et al. (2011) Alleged Cases of Vaccine Encephalopathy Rediagnosed Years Later as Dravet Syndrome. Pediatrics.
- 25 – Griffin et al. (1990) Risk of Seizures and Encephalopathy After Immunization With the Diphtheria-Tetanus-Pertussis Vaccine. JAMA.


