
Flawed reasoning: The claim implies vaccinated people are at greater risk of neurological problems compared to unvaccinated people. This is incorrect, as risks reported in the study were calculated by comparing people who received one type of COVID-19 vaccine to another group receiving a different COVID-19 vaccine. The study didn’t compare the risk of neurological events post-vaccination to that of unvaccinated people.

FULL CLAIM: “In summary, a shocking 31.2% of respondents to this large dataset sustained neurologic injury after two injections with verified data in health registries. Most of the risk estimates indicate the safety profile is unacceptable”; “Nearly 1 in 3 COVID-19 Vaccine Recipients Suffered Neurological Side Effects: Study”; “NEURO-COVAX: Italian Network Finds Neurological Side Effects Very Common after COVID-19 Vaccination”
REVIEW
In late October 2023, a claim that a study found “nearly 1 in 3 COVID-19 vaccine recipients suffered neurological side effects” made the rounds on the Internet. The claim originates from an Epoch Times article, which is in turn based on a Substack article by cardiologist Peter McCullough. McCullough has figured prominently in the spread of COVID-19 misinformation. The Epoch Times posted the same claim on Instagram, receiving more than 11,000 likes.
In his Substack article, McCullough cited a study by researchers in Italy, which surveyed vaccinated people to measure the number of neurological adverse events occurring after COVID-19 vaccination. He claimed the study showed “a shocking 31.2% of respondents to this large dataset sustained neurologic injury after two injections with verified data in health registries” and called the safety profile of the COVID-19 vaccines “unacceptable”.
The article concluded by promoting a “Base Spike Detoxification (BSD)” protocol he claimed would help with vaccine injury. The protocol calls for the use of nattokinase and bromelain, among other things. The Wellness Company, of which McCullough is chief scientific officer, has also promoted the same protocol and markets supplements containing nattokinase and bromelain.
McCullough’s claims were also repeated in the Epoch Times article, unquestioned.
However, the study didn’t find that COVID-19 vaccines had an “unacceptable” safety profile and significant details about the way the study calculated risk weren’t provided in the McCullough and Epoch Times article. We explain below.
What did the study do and what did it find?
The study included people aged 18 and above who received any COVID-19 vaccine in the region of Lombardy in Italy between 7 and 16 July 2021.
Participants were provided a questionnaire asking for information about their vaccination and the symptoms they experienced after vaccination. Questionnaires were given to each participant twice per dose of vaccine: the first for acute adverse events (defined as symptoms that started within the first 15 to 30 minutes after vaccination), and the second for subacute adverse events (defined as symptoms occurring within the first two weeks after vaccination).
Thus, with a single-dose vaccine like the adenovirus vector vaccine, one participant could complete up to two questionnaires, while with double-dose vaccines like the mRNA vaccines, one participant could complete up to four questionnaires.
Approximately 19,000 participants and more than 67,000 questionnaires were included in the study’s analysis. Apart from ascertaining whether participants had been hospitalized for any symptoms post-vaccination via digital healthcare records, the researchers didn’t examine medical records to verify participants’ responses. Therefore, the study’s results are largely based on participants’ self-reports of symptoms.
The researchers acknowledged that the self-reports were a limitation of the study, stating “our results should be interpreted with caution because of a possible overestimation of neurological events resulting from the self-reported symptoms”.
The responses showed that approximately a third of participants reported experiencing at least one neurological complication. More than half of these complications were headaches; sleepiness was the runner-up, comprising more than a third of all reports of neurological complications (see Figure 1 below). The responses also indicated that these were relatively brief and self-limiting. Headaches were reported to last approximately a day, while sleepiness lasted a week (see Figure 2 below).
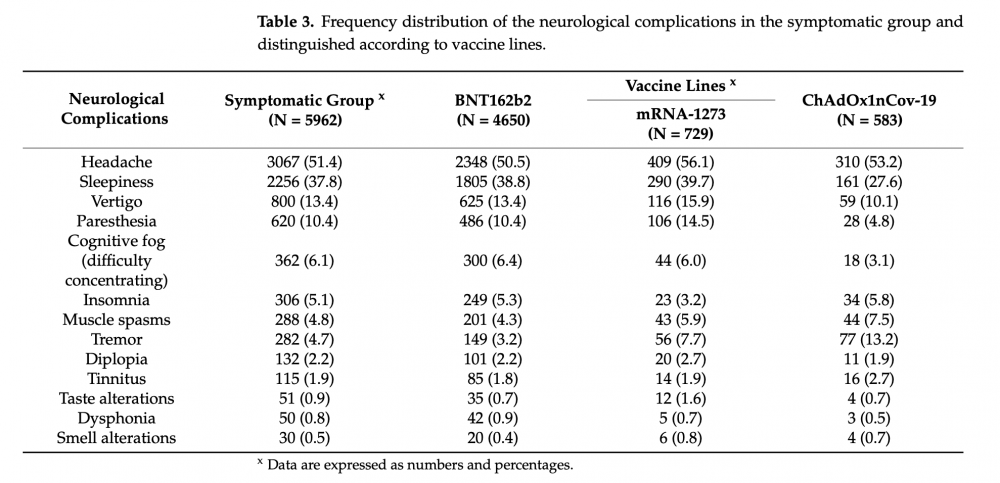
Figure 1 – Table showing the range of neurological symptoms reported by participants in the study.
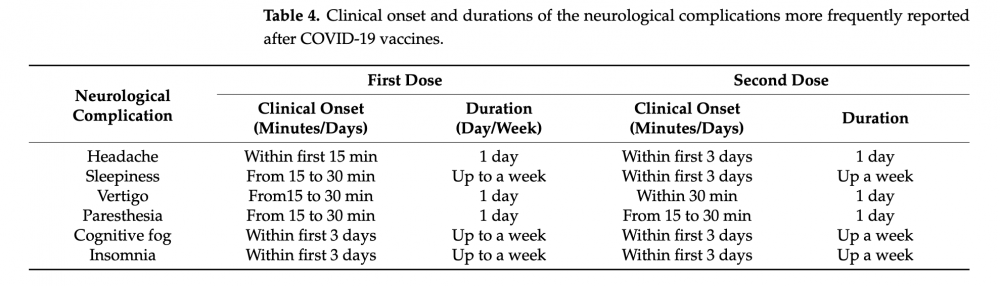
Figure 2 – Table showing when the most frequently reported neurological symptoms began and the duration the symptom lasted.
The researchers reported that they “registered no severe neurological and/or non-neurological complications and/or death following COVID-19 vaccination”.
The study didn’t find COVID-19 vaccination to be associated with serious health concerns
In his Substack article, McCullough cited the study’s findings as an argument against COVID-19 vaccination, calling the COVID-19 vaccines “ill-advised”. This implies that the risks reported in the study were calculated by comparing vaccinated and unvaccinated people.
This isn’t true. Instead, the risk for a particular complication following one type of COVID-19 vaccine was calculated relative to another COVID-19 vaccine. For example, the risk for tremors after receiving the AstraZeneca-Oxford COVID-19 adenovirus vector vaccine was expressed relative to the Pfizer-BioNTech mRNA vaccine.
This information is also present in the study’s abstract that both McCullough and The Epoch Times cited:
“[…] we found an increased risk for ChAdOx1nCov-19 of tremors (vs. BNT162b2, OR: 5.12, 95% CI: 3.51–7.48); insomnia (vs. mRNA-1273, OR: 1.87, 95% CI: 1.02–3.39); muscle spasms (vs. BNT162b2, OR: 1.62, 95% CI: 1.08–2.46); and headaches (vs. BNT162b2, OR: 1.49, 95% CI: 0.96–1.57). For mRNA-1273, there were increased risks of parethesia (vs. ChAdOx1nCov-19, OR: 2.37, 95% CI: 1.48–3.79); vertigo (vs. ChAdOx1nCov-19, OR: 1.68, 95% CI: 1.20–2.35); diplopia (vs. ChAdOx1nCov-19, OR: 1.55, 95% CI: 0.67–3.57); and sleepiness (vs. ChAdOx1nCov-19, OR: 1.28, 95% CI: 0.98–1.67)” [emphasis added]
Therefore, contrary to McCullough’s implication, the study cannot tell us anything about the difference in risk between those who got vaccinated against COVID-19 and those who didn’t.
Health Feedback reached out to the study’s authors for comment.
In an email, the study’s corresponding authors, neurologists Maria Salsone and Luigi Ferini-Strambi, clarified that “we are in favor of the COVID-19 vaccination”. They stressed that “the neurological effects reported […] are frequent but of minor severity such as headaches, thus these may be considered as minor neurological complications”. Furthermore, these symptoms were “reversible in few days, at most in a week”.
They added that no participant reported severe neurological complications, such as Guillain-Barré syndrome, Bell’s palsy, transverse myelitis and encephalitis, and none had been “hospitalized and/or died for severe complications related to COVID-19 vaccination”.
“Overall, given the large sample size and the clinical entity of the neurological symptoms, we strongly encourage the COVID-19 vaccination,” they concluded.
It should be noted that headache is among the most common side effects of COVID-19 vaccination, along with other flu-like symptoms like tiredness. However, these side effects are benign and aren’t associated with long-term repercussions. The study’s findings, as we pointed out in the previous section, also indicate that most of these side effects were self-limiting, resolving after anywhere between a day to a week.
Therefore, McCullough’s description of relatively short-lived headaches and sleepiness as “neurologic injury” that presents an “unacceptable” safety profile isn’t justified by the study’s findings.
Getting COVID-19 is known to increase the risk of potentially serious medical problems. Studies have reported an elevated risk of developing blood clotting disorders and cardiovascular problems, for starters[1-6]. While COVID-19 vaccination does carry risks as well, these are smaller than the risks posed by COVID-19 itself. COVID-19 vaccines significantly reduce the risk of severe disease and death. On balance, their benefits outweigh their risks.
REFERENCES
- 1 – Raisi-Estabragh et al. (2022) Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank. Heart.
- 2 – Knight et al. (2022) Association of COVID-19 With Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales. Circulation.
- 3 – Hippisley-Cox et al. (2021) Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ.
- 4 – Xie et al. (2022) Long-term cardiovascular outcomes of COVID-19. Nature Medicine.
- 5 – Patone et al. (2022) Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation.
- 6 – Barda et al. (2021) Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. New England Journal of Medicine.


