
Unsupported: There’s no reliable evidence showing that DNA in vaccines integrates into our DNA or increases the risk of cancer. There are, in fact, several vaccines predating COVID-19 vaccines containing DNA, such as the chickenpox vaccine. These have been shown to be safe.

FULL CLAIM: “You can now sue the mRNA COVID vaccine manufacturers for damages and the FDA is required to take the COVID vaccines off the market. Why? Adulteration. The plasmid bioactive contaminant sequences were NOT pointed out to the regulatory authorities.”; “Health Canada on Thursday confirmed the presence of DNA contamination in Pfizer COVID-19 vaccines and also confirmed that Pfizer did not disclose the contamination to the public health authority”
REVIEW
A preprint posted on 20 October 2023 to the OSF preprint server claimed that DNA fragments were present in certain lots of the Pfizer and Moderna COVID-19 vaccines. It also claimed the amount of residual DNA correlated with the number of serious adverse events associated with particular vaccine lots, suggesting that the adverse events may have been caused by residual DNA.
This is the latest spin on an earlier claim that DNA contamination in COVID-19 mRNA vaccines posed a cancer risk, which went viral in June 2023. This claim was based on an April 2023 preprint, claiming to show that the Pfizer COVID-19 mRNA vaccines contained a DNA sequence from the SV40 virus. SV40 virus has been found to cause cancer in some animals like hamsters. The April preprint was already discussed at length in an earlier review. Based on the available evidence, Health Feedback concluded the claims were unsubstantiated.
Incidentally, an Epoch Times article that appeared just one day before the October 2023 preprint claimed that the Canadian drug regulator Health Canada “says Pfizer did not disclose the presence of the Simian Virus 40 (SV40) DNA sequence”, citing the claims from the April preprint. The Epoch Times has published misinformation about COVID-19 and vaccines on numerous occasions.
The two preprints, combined with the Epoch Times article, renewed discussions on social media about the potential implications for residual DNA in vaccines.
Unsurprisingly, social media posts from individuals and groups known to be opposed to vaccination—such as this tweet by entrepreneur Steve Kirsch, this article by Children’s Health Defense, and this article by Rebel News—seized on the article to claim that residual DNA contamination wasn’t disclosed by vaccine manufacturers and imply that the residual DNA in the COVID-19 vaccines was harmful. Some related posts were tagged with the hashtag #PlasmidGate—implying that the preprint’s findings about residual DNA in COVID-19 vaccines were revelatory and a scandal.
To help readers understand whether these claims are supported by the evidence, this review will discuss the work performed in the October 2023 preprint, the implications of its findings, and whether there’s reason to believe residual DNA in the vaccines poses a significant health concern, as some social media posts implied.
What did the authors of the October 2023 preprint do?
The October 2023 preprint was a follow-up of the results reported in the April 2023 preprint by McKernan et al. It set out to measure the level of DNA in several vials of Pfizer and Moderna COVID-19 vaccines, belonging to different lots used in Canada.
Residual DNA present in the COVID-19 mRNA vaccine is a result of the process used to make the vaccine. The Pfizer COVID-19 mRNA vaccine is manufactured by mass producing the genetic material for the SARS-CoV-2 spike protein in the bacterium Escherichia coli. This is accomplished by placing the spike protein’s genetic material into a plasmid, which is a circular DNA molecule, which is replicated by E. coli.
E. coli divides every 20 minutes, provided laboratory conditions are optimal, meaning that very large amounts of plasmid can be generated in a relatively short amount of time[3].
The DNA is then harvested from the bacteria and cut so that the segment containing the spike protein’s genetic material is isolated so that it can be transcribed into mRNA.
The authors of the preprint used two different methods for measuring DNA levels: quantitative PCR—which is also the gold standard for detecting SARS-CoV-2 infection—and fluorometry, which uses fluorescent markers that bind to nucleic acids like DNA. The main findings of the preprint centered on the measurement of the spike protein DNA inserted into a plasmid, as well as the DNA marking the origin of replication (ori) on the plasmid.
The authors also wanted to explore the question of whether residual DNA levels correlated with the number of adverse events in the U.S. Vaccines Adverse Event Reporting System.
Per the preprint’s Method section, they collected VAERS data related to the vaccine lots that they had analyzed, although they limited the reports to only those from outside the U.S. The reason given for limiting their analysis only to reports outside the U.S. was that they wished to reduce the level of confounding as a result of differences in adverse event reporting rate due to potential underreporting and mandatory reporting requirements within and outside the U.S. No reason was given for why the authors considered data from outside the U.S. to be less affected by differences in reporting rate.
The preprint didn’t find concerning levels of residual DNA in COVID-19 vaccines
One of the preprint’s key takeaways is that the levels of residual DNA detected in the vaccine vials using qPCR were actually well below the World Health Organization and U.S. Food and Drug Administration’s recommended limit of 10 ng DNA/dose. The preprint acknowledged this, stating that “qPCR residual DNA content in all vaccines were below [guidelines set by FDA and WHO of 10 ng/dose]”.
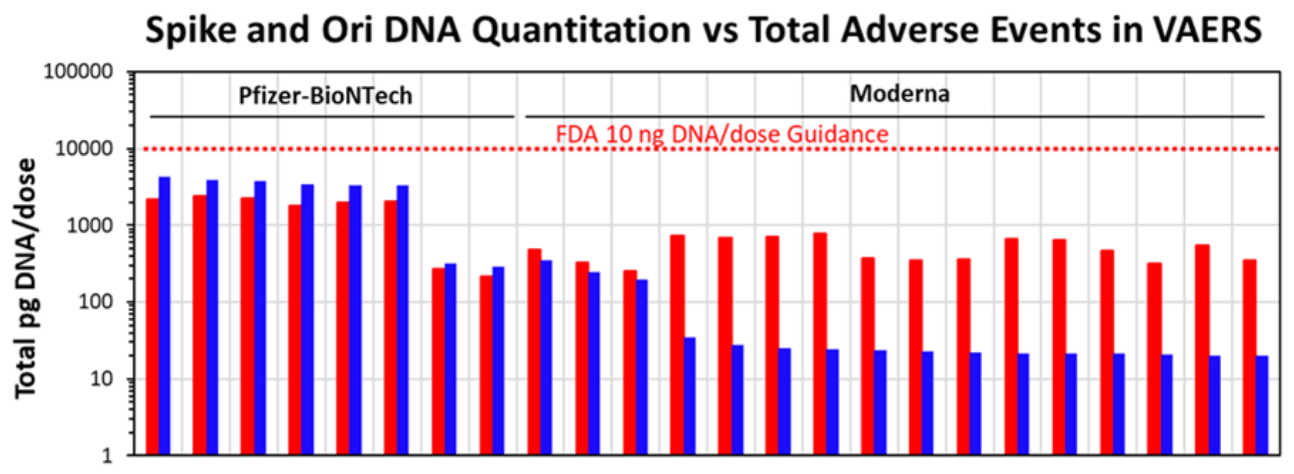
Figure 1 – The quantity of spike DNA (red) and plasmid DNA (blue) corresponding to the origin of replication (the region on the plasmid where DNA replication begins) in each of the vials tested. One picogram (pg) is a thousandth of a nanogram. Note that the y-axis is on a log scale, not a linear scale.
However, the measurement of DNA levels using fluorometry seemed to greatly contrast with the findings of the qPCR tests. The authors reported that this method showed the quantity of DNA in the vaccines exceeded the WHO and FDA guidelines by more than 188 to 509 times.
While this seems alarming, the caveat here is that fluorometry is less specific than qPCR, as the fluorescent marker that the authors used can also bind RNA as well as DNA. This limitation is important to account for, given that the vaccine vials contained a mix of DNA and RNA.
In this article, David Gorski, a surgical oncologist and cancer researcher at Wayne State University, pointed out that the vaccine samples had to be treated with high heat at 95°C before DNA quantification. The heat would have disrupted the lipid nanoparticles encasing the RNA, releasing the RNA into the solution. “When there is a lot more RNA than [double-stranded DNA], even a highly selective assay could be affected by the RNA,” he concluded.
Mikael Niku, a senior university lecturer at Helsinki University who studies host-microbial interactions, wrote to Kevin McKernan, one of the preprint’s authors, on X/Twitter, raising the same issue: “The fluorometry kit you used is NOT specific for DNA. Biotium tech support says it’s only ‘10X or more selective’ for DNA over RNA and you should use it for *clean* dsDNA preparations”.
One of the preprint’s authors, Kevin McKernan, responded to this criticism by pointing to how Pfizer had used fluorometry to measure RNA levels in their vaccine.
Niku countered that “Fluorometry is completely valid when you’re measuring the concentration of the MAJOR nucleic acid of the solution, which obviously is RNA in [Pfizer’s] case”.
Simply put, the apparently massive amount of DNA detected by fluorometry could have been due to the high level of free RNA in the sample—as expected in an mRNA vaccine—and not DNA.
Gorski suggested that the authors could have checked to see if this was the case by treating the samples with the enzyme RNase, which breaks down RNA, and then measuring the level of DNA using fluorometry afterwards. But the authors didn’t report doing so, thus we cannot rule out this possibility.
Next, the authors’ attempt to correlate the number of serious adverse event reports from VAERS with the amount of DNA detected in the vaccine lots tested also raises some questions.
As Gorski pointed out, there were too few data points (four or five in most cases) to draw a reliable correlation in the first place.
For example, the graph showing Pfizer data is interpreted as showing that the proportion of serious adverse events rose with the amount of DNA detected by qPCR, even though one shouldn’t trust a correlation with so few data points (Figure 2). But in the case of Moderna, having more spike DNA is interpreted with a line showing more SAEs, while having more DNA from the plasmid’s origin of replication (ori) is interpreted with a line showing fewer SAEs.
So in addition to not being reliable, as they are based on too few data points, these trend lines are inconsistent with one another.
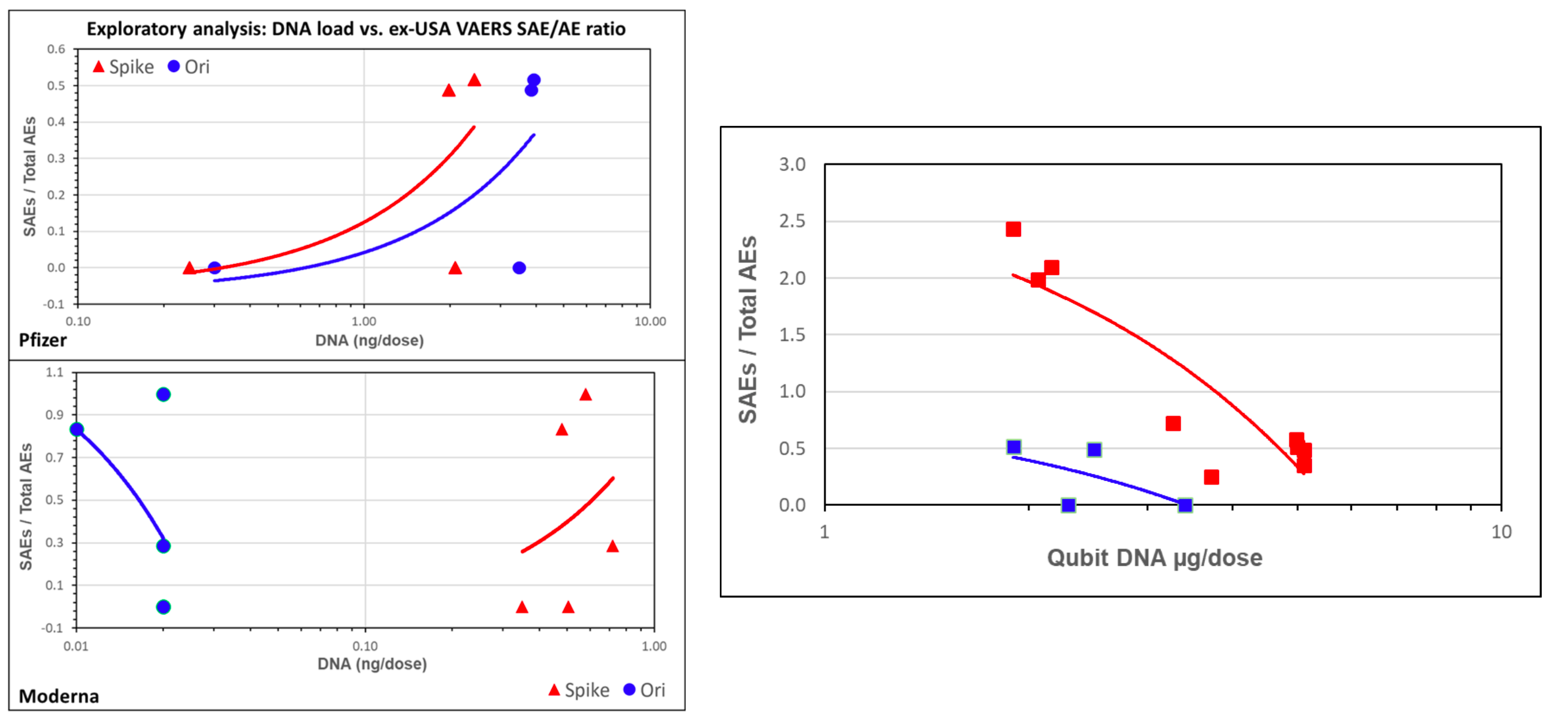
Figure 2 – Figures allegedly showing correlations of the amount of DNA detected by qPCR (left) and Qubit fluorometric assay (right) with the number of serious adverse events (as a proportion of total adverse events). Left: The red line corresponds to spike DNA while the blue line corresponds to plasmid DNA. The top graph shows results from the Pfizer vaccine and the bottom graph shows results from the Moderna vaccine. Right: The blue line corresponds to Pfizer, the red line to Moderna. Source: Speicher et al.
And the correlation that used fluorometry-derived DNA measurements suggested that the more the residual DNA present in the vaccine, the fewer the serious adverse events. No acknowledgement of this result was made in social media posts that relied on the preprint to claim residual DNA would be harmful.
We reached out to the authors of the preprint with questions about the methods they used via email. In his email response, David Speicher, the corresponding author of the preprint and a senior research associate at the University of Guelph, didn’t answer our questions in writing, offering instead to do so via a Zoom call. He added that the authors would “take [our] interesting questions into consideration for clarification in future versions of the manuscript”.
Regulatory agencies were aware of residual DNA in COVID-19 vaccines prior to the preprint, didn’t find evidence for concern
Despite the assumptions made by certain social media posts, concerns over the potential health effects of DNA in vaccines are neither new nor unknown to regulatory agencies, as various publications predating the COVID-19 pandemic demonstrate.
Based on a publicly available document submitted by BioNTech to the European Medicines Agency (EMA), dated 19 February 2021, we know that part of the vaccine manufacturing process involves treating batches of the resulting RNA with an enzyme called DNase. This enzyme digests DNA, breaking it into fragments. Thus both BioNTech and the EMA were aware of DNA impurities in the vaccine and of steps taken to reduce this impurity.
It’s incorrect to claim that regulatory agencies were unaware of residual DNA in the COVID-19 mRNA vaccines until now, as the same document submitted to the European Medicines Agency indicated that residual DNA was among the impurities quantified by the manufacturer (“Process- and product-related impurities including host cell genomic DNA, RNA, proteins, endotoxins, bioburden and plasmid isoforms, for the plasmid DNA, are routinely quantified”).
Emails released by The Epoch Times also show that Health Canada was “aware of the presence of residual plasmid DNA as a process-related impurity during review and prior to the authorisation of the mRNA COVID-19 vaccines”.
However, this part of Health Canada’s response didn’t appear in the Epoch Times article, which provided room for the inaccurate claim that regulators were unaware of residual DNA in the COVID-19 vaccines, put about by Kirsch, Children’s Health Defense, Rebel News, and others.
In its response to The Epoch Times, Health Canada also added that “the release testing data for every COVID-19 vaccine lot released into the Canadian market were reviewed and deemed to meet the requirement approved by Health Canada”.
In brief, the claim that regulatory agencies like Health Canada hadn’t known about residual DNA in COVID-19 vaccines or that vaccine manufacturers didn’t disclose the presence of residual DNA in the vaccines is inaccurate.
We reached out to Health Canada and the FDA for comment regarding these claims.
In response to our questions about DNA contamination in the COVID-19 vaccines, Health Canada said in a statement:
“As a regulator, Health Canada sets quality standards and requirements for manufacturers to follow, including providing comprehensive and detailed information about the vaccine itself, and about the manufacturing process. In the manufacture of any vaccine, residual elements that are part of the standard manufacturing process may remain. There are strict limits and controls for the presence of these residual fragments to ensure that there is no effect on the safety or effectiveness of the vaccine.
The Pfizer-BioNTech COVID-19 vaccine does not contain simian virus 40 (SV40). The presence of the SV40 promoter enhancer sequence is not the same as the presence of the whole virus itself.
The SV40 promoter enhancer sequence was found to be a residual DNA fragment in Pfizer-BioNTech COVID-19 vaccine. The fragment is inactive, has no functional role, and was measured to be consistently below the limit required by Health Canada and other international regulators.”
It also added that “[a]ny claims that the presence of the SV40 promoter enhancer sequence is linked to an increased risk of cancer are unfounded”. We have provided Health Canada’s full statement at the end of our review.
The FDA informed us via email that our questions had been forwarded to the Center for Biologics Evaluation and Research (CBER), which regulates biologically derived products including vaccines, and would follow up with a response as soon as possible. We will update this review if new information becomes available.
No evidence that residual DNA in COVID-19 mRNA vaccines poses a health risk
Much talk of the potential health effects of residual DNA in the vaccines has revolved around the possibility that the DNA could integrate into our genome and cause diseases like cancer. However, this isn’t substantiated by evidence.
Health Canada’s response to The Epoch Times stated that “the DNA plasmid used for the Pfizer vaccine production is linearised, degraded, and reduced in quantity through additional steps. There is no peer-reviewed evidence that linearised or fragmented DNA is capable of translocating to the nucleus of cells”. This part of the response wasn’t included in the Epoch Times article.
In a tweet, McKernan suggested that even small bits of DNA from regulatory elements, like the SV40 promoter, could still pose a risk of DNA integration, citing FDA guidance[4]. However, this misses significant context given in the guidance:
“In evaluating the potential harm of plasmid integration, it should be noted that the risk of introducing plasmids with strong regulatory regions into the host genome far exceeds that associated with random point mutations […] In this context, sections of DNA as short as 7 bp can affect rates of integration or recombination. Examples include the VDJ recombination signal sequence and related sequences, chi-like elements and minisatellites, ALU sequences, a recombinase signal present in hepatitis B and mammalian genomes, and topoisomerase II recognition sites.” [emphasis added]
Several of these given examples all have to do with regulatory elements that are very short to begin with. For example, V(D)J recombination sequences are between seven to nine base pairs long[5]. Minisatellites range between 10 to 50 base pairs. The SV40 promoter, on the other hand, spans more than 300 base pairs. It’s doubtful whether it would still retain biological activity after it was broken down into smaller pieces.
Moreover, even if residual DNA were to make it into our cells, there’s also no evidence indicating this would lead to integration. Marc Veldhoen, an immunologist and professor at the University of Lisbon, took to X/Twitter to highlight the fact that there are already a number of vaccines in use that contain DNA, such as the COVID-19 adenovirus vector vaccines, as well as the chickenpox vaccine (the virus for chickenpox is a DNA virus). There’s no evidence that these vaccines are associated with a greater risk of developing cancer.
He added:
“Like DNA or RNA vaccines, vaccines using attenuated or killed pathogens work from a similar principle. The DNA/RNA gets into your cells, and protein from the pathogen is made. Important(sic), DNA/RNA vaccines cannot amplify nor do they generate infectious material.”
In all these cases, DNA would make it into our cells. However, our cells have multiple ways to detect foreign DNA and destroy it, since our immune system sees foreign DNA as a sign of infection[6-8]. This would eventually lead the affected cells to die by programmed cell death (apoptosis) and the removal of the cell, proteins, DNA, and RNA left behind.
“So no, even with scare stories about SV40 enhancers, the DNA or RNA does not get into the nuclei, it certainly does not integrate, the cell dies. It detects DNA or RNA, and it dies. It makes foreign protein, and it dies. i.e.; no matter what, the cell dies,” he concluded.
Health Canada’s statement in response to Health Feedback’s questions about DNA contamination in COVID-19 vaccines
“Health Canada initially authorized the Pfizer-BioNTech COVID-19 mRNA vaccine in December 2020 and subsequently has authorized updated versions, including the most recent vaccine targeting the XBB Omicron subvariant in September 2023. Each assessment included a determination that the vaccine met the Department’s stringent regulatory safety, efficacy and quality requirements for use in Canada.
As a regulator, Health Canada sets quality standards and requirements for manufacturers to follow, including providing comprehensive and detailed information about the vaccine itself, and about the manufacturing process. In the manufacture of any vaccine, residual elements that are part of the standard manufacturing process may remain. There are strict limits and controls for the presence of these residual fragments to ensure that there is no effect on the safety or effectiveness of the vaccine.
The Pfizer-BioNTech COVID-19 vaccine does not contain simian virus 40 (SV40). The presence of the SV40 promoter enhancer sequence is not the same as the presence of the whole virus itself.
The SV40 promoter enhancer sequence was found to be a residual DNA fragment in Pfizer-BioNTech COVID-19 vaccine. The fragment is inactive, has no functional role, and was measured to be consistently below the limit required by Health Canada and other international regulators.
Any claims that the presence of the SV40 promoter enhancer sequence is linked to an increased risk of cancer are unfounded. There is also no evidence to support that the presence of the full SV40 in any vaccine increases the risk of cancer or the acceleration of cancer in individuals.
Health Canada continues to monitor the COVID-19 vaccines to ensure that they continue to meet the highest standards for safety, effectiveness and quality and that their benefits continue to outweigh any potential risks.”
UPDATE (28 October 2023):
We updated our review to include responses from the preprint’s corresponding author, Health Canada, and the FDA. This information was added to the twenty-seventh and thirty-sixth paragraphs. The full statement by Health Canada was also appended to the end of the review.
REFERENCES
- 1 – Girardi et al. (1962) Development of Tumors in Hamsters Inoculated in the Neonatal Period with Vacuolating Virus, SV40. Experimental Biology and Medicine.
- 2 – Cicala et al. (1993) SV40 induces mesotheliomas in hamsters. American Journal of Pathology.
- 3 – Gibson et al. (2018) The distribution of bacterial doubling times in the wild. Proceedings of the Royal Society of London. Series B.
- 4 – Klinman et al. (2010) FDA Guidance on Prophylactic DNA Vaccines: Analysis and Recommendations. Vaccine.
- 5 – Bassing et al. (2000) Recombination signal sequences restrict chromosomal V(D)J recombination beyond the 12/23 rule. Nature.
- 6 – Briard et al. (2020) DNA Sensing in the Innate Immune Response. Physiology.
- 7 – Paludan and Bowie. (2013) Immune Sensing of DNA. Immunity.
- 8 – Motwani et al. (2019) DNA sensing by the cGAS–STING pathway in health and disease. Nature Reviews Genetics.


