
Misleading: Firstly, the standard of safety testing at the time that thalidomide was approved in the 1950s was much lower than the present standard. COVID-19 vaccine candidates are tested for safety across multiple stages of clinical trials in thousands of people, and are required to demonstrate a high level of safety before being approved for use. Equating the two is highly misleading. Secondly, thalidomide was not a “rapid approved” drug. The concept of rapid drug approval as we understand it today did not exist in the 1950s.

FULL CLAIM: “Thalidomide was a rapid approved drug introduced in 1957 to counteract nausea and insomnia in pregnant women. It was marketed in 50 countries before being withdrawn in 1962 due to malformations in newborns. Be very careful with what’s coming.”
REVIEW
The relatively short timeline in which COVID-19 vaccine candidates have reached the advanced stages of clinical trials have led many members of the public to express concerns about their safety. Following the announcement of the U.K.’s approval for Pfizer’s COVID-19 vaccine candidate on 2 December 2020, claims that drew parallels between thalidomide and COVID-19 vaccines went viral on social media platforms, with the hashtag #thalidomide appearing in the top ten entries of U.K.’s Twitter trends, as reported by The Herald.
One tweet that was published on 30 November 2020 and went viral on Facebook in the form of a screenshot (archived here) states, “Thalidomide was a rapid approved drug introduced in 1957 to counteract nausea and insomnia in pregnant women. It was marketed in 50 countries before being withdrawn in 1962 due to malformations in newborns. Be very careful with what’s coming.”
Thalidomide is a drug that was developed in Germany in the 1950s. It was first prescribed as a sedative and then to treat morning sickness in pregnant women as well. At that time, drug testing was less stringent and thorough. For instance, drugs that were prescribed to pregnant women were not necessarily tested for toxic effects on a developing fetus (teratogenicity). Scientists did not know at the time that certain drugs could pass through the placenta and thereby affect the fetus. In fact, it was cases of thalidomide-induced birth defects that led scientists to the realization that drugs could cross the placenta and harm the fetus[1].
According to the U.K. Science Museum:
“During early testing, researchers at the company found that it was virtually impossible to give test animals a lethal dose of the drug (based on the LD50 test). Largely based on this, the drug was deemed to be harmless to humans. Thalidomide was licensed in July 1956 for over-the-counter sale (no doctor’s prescription was needed) in Germany.”
However, reports of birth defects associated with the drug grew over time:
“As the drug was traded under so many different names in 49 countries, it took five years for the connection between thalidomide taken by pregnant women and the impact on their children to be made. A UK Government warning was not issued until May 1962.
One reason why researchers and doctors were slow to make this connection was due to the wide range of changes to foetal development. Limbs, internal organs including the brain, eyesight and hearing could all be affected.
Later, they found that the impact on development was linked to when during pregnancy the drug was taken, and effects only occurred between 20 and 37 days after conception. After that, thalidomide had no effect on the foetus.
Another reason why it took so long to establish the link to thalidomide was that some of the damage caused by the drug was very similar to certain genetic conditions that affect the upper or lower limbs.”
Withdrawal of thalidomide from the market began in 1961, although the drug is still available today through prescription for the treatment of certain diseases, such as leprosy.
While thalidomide does cause birth defects, it was never subjected to the same level of rigor in safety testing that is being used for the COVID-19 vaccine candidates (see Figure 1). Indeed, it was thalidomide that led many countries, including the U.S., to tighten regulations and implement more stringent testing standards for drugs, building the methodological foundation for the clinical trials that we see today. It is therefore misleading to equate COVID-19 vaccines to thalidomide. In addition, thalidomide was not a “rapid approved” drug as claimed, as the concept of rapid approval or “fast tracking” of drug approval, in the sense that we understand it today, did not exist in the 1950s. For example, the earliest programs for expedited approval by the U.S. Food and Drug Administration (FDA) only began in the 1980s[2].
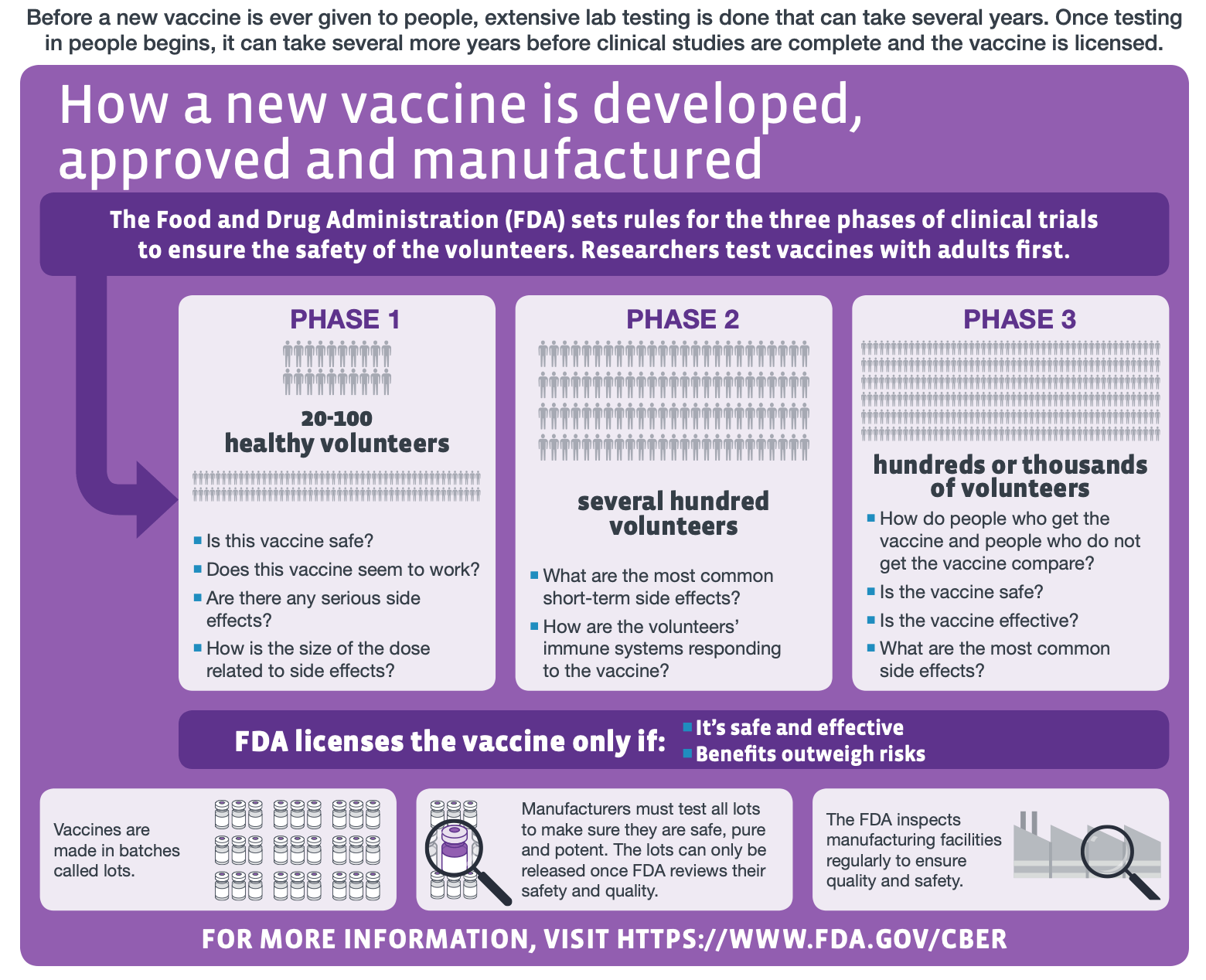
Figure 1—The different stages of human clinical trials in vaccine development (modified from the original graphic by the U.S. Centers for Disease Control and Prevention).
Although some COVID-19 vaccine candidates have sought approval in record time, this does not mean that corners have been cut in their testing. Rather, the conventionally lengthy development time for a vaccine points to barriers, such as bureaucratic red tape, which have been removed given the exceptional circumstances created by the pandemic. As Mark Toshner, director of Translational Biomedical Research at Cambridge University, points out in this article in The Conversation:
“So next time somebody expresses concern at the astonishing speed the vaccine trials have happened at, point out to them that ten years isn’t a good thing, it’s a bad thing. It’s not ten years because that is safe, it’s ten hard years of battling indifference, commercial imperatives, luck and red tape. It represents barriers in the process that we have now proved are ‘easy’ to overcome. You just need unlimited cash, some clever and highly motivated people, all the world’s trial infrastructure, an almost unlimited pool of altruistic, wonderful trial volunteers and some sensible regulators.
[…] Safety has not been compromised. All trials have been through the correct “phases” or process of any normal drug or vaccine. Hundreds of thousands of the very best of us volunteered and had an experimental vaccine. The world watched so closely that when a single person fell ill, we were all debating it.
To date, there has not been a single associated death related to COVID vaccines and only a handful of potentially serious events.”
So far, two COVID-19 vaccine candidates, one from Pfizer and the other from Moderna, have been submitted to the FDA for Emergency Use Authorization (EUA). Both vaccine candidates have reached Phase 3 clinical trials and have not demonstrated serious adverse effects in earlier phases[3-5]. The FDA explains the EUA process for a vaccine, which demonstrates the emphasis on vaccine safety:
“For an EUA to be issued for a vaccine, for which there is adequate manufacturing information to ensure quality and consistency, FDA must determine that the known and potential benefits outweigh the known and potential risks of the vaccine.
[…] From a safety perspective, FDA expects an EUA submission will include all safety data accumulated from phase 1 and 2 studies conducted with the vaccine, with an expectation that phase 3 data will include a median follow-up of at least 2-months (meaning that at least half of vaccine recipients in phase 3 clinical trials have at least 2 months of follow-up) after completion of the full vaccination regimen. In addition, FDA expects that an EUA request will include a phase 3 safety database of well over 3,000 vaccine recipients, representing a high proportion of participants enrolled in the phase 3 study, who have been followed for serious adverse events and adverse events of special interest for at least one month after completion of the full vaccination regimen.”
FDA officials Stephen Hahn and Peter Marks stated:
“We are committed to expediting the development of COVID-19 vaccines, but not at the expense of sound science and decision making. We will not jeopardize the public’s trust in our science-based, independent review of these or any vaccines. There’s too much at stake.”
READ MORE
This article by epidemiologist Gideon Meyerowitz-Katz explains the process of safety testing and monitoring for COVID-19 vaccines.
REFERENCES
- 1 – Hill and Kleinberg. (1984) Effects of drugs and chemicals on the Fetus and Newborn (First of Two Parts). Mayo Clinic Proceedings.
- 2 – Kesselheim et al. (2015) Trends in utilization of FDA expedited drug development and approval programs, 1987-2014: cohort study. British Medical Journal.
- 3 – Jackson et al. (2020) An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. New England Journal of Medicine.
- 4 – Anderson et al. (2020) Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. New England Journal of Medicine.
- 5 – Walsh et al. (2020) Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine.


