
Introduction
According to an estimate published by the World Health Organization in April 2023, infertility affects roughly one in six (17.5%) adults of reproductive age worldwide. Factors causing or contributing to infertility can occur before birth or develop later in life and are so varied and complex that, in many cases, a cause can’t be identified.
One of the known leading causes of infertility is hormone imbalance. Hormones are chemical messengers that regulate all essential biological functions in the body, including reproduction, development, and metabolism. Although they circulate in the body at very low levels, subtle changes in hormone concentrations can produce powerful effects. For this reason, even small disruptions in hormone signaling can result in serious health problems.
In the 1960s and 1970s, researchers found that certain chemicals in pesticides and medicines interfered with hormones, causing reproductive problems in animals and humans. But it wasn’t until 1991 that the Wingspread Conference introduced the term endocrine disruptor to refer to this type of chemical, producing the first consensus statement about the potential harm of these substances to human health.
Since then, chemicals with endocrine-disrupting potential have been identified in a variety of products of everyday use, including pesticides, fungicides, plastics, solvents, lubricants, electronics, and textiles[1].
Researchers are only beginning to understand the effects that these chemicals might have on people’s health. They know, however, that exposure to endocrine disruptors during pregnancy and early childhood can be particularly harmful, because it is at these stages that all organs develop, a process that relies heavily on hormones.
One group of endocrine disruptors that has received much public attention is phthalates, a group of synthetic chemicals with broad industrial applications that has become ubiquitous in people’s everyday lives. In recent years, phthalates have been blamed for a variety of health problems, including infertility and, more recently, “shrinking penises”. But is there scientific support for these claims?
In this article, we will analyze current knowledge about these and other potential effects of phthalates on human reproduction.
How are people exposed to phthalates?
Phthalates are divided into two broad categories according to their mass: high [molecular weight] phthalates and low [molecular weight] phthalates, each with different properties and applications.
High phthalates are often called plasticizers because they are mainly used to manufacture polyvinyl chloride (PVC), making it more flexible and durable. PVC is among the most widely used plastics worldwide as part of construction materials, wall and floor coverings, toys, food packaging, and medical devices. Some examples of common high phthalates are bis(2-ethylhexyl) phthalate (DEHP), butyl benzyl phthalate (BBP), diisononyl phthalate (DiNP), diisodecyl phthalate (DiDP), and di(2-propylheptyl) phthalate (DPHP).
Low phthalates are used as solvents and to make scents last longer in cosmetics, personal care products, and fragrances. Some are also approved as additives in foods and to help slow the release of drugs in medications[2]. The most common low phthalates include dibutyl phthalate (DBP), dimethylphthalate (DMP), and diethylphthalate (DEP).
But phthalates, especially low phthalates, aren’t strongly bound to the materials they are part of. This means they can leach out of these products into the environment, exposing people to their potential effects through several different pathways.
Eating and drinking food and water that has been in contact with phthalates during production and packaging is considered a major route of exposure[3-5]. In addition, phthalates can be absorbed through the skin and be inhaled as steam or in particles attached to dust[6] (Figure 1).
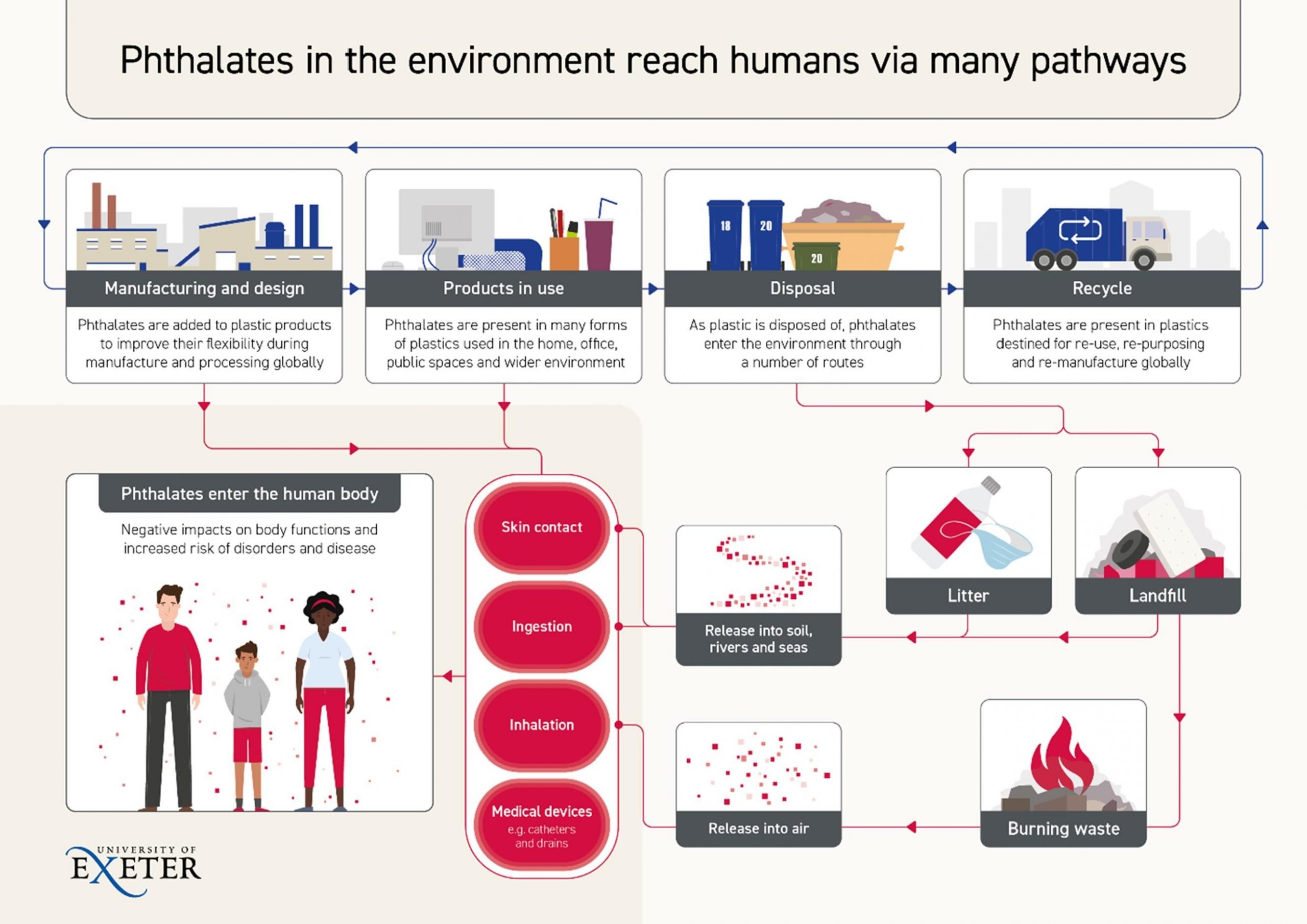
Figure 1 – Main routes of phthalate exposure in humans. Phthalates can be released into the environment during the production, use, and disposal of phthalate-containing products. Once released, phthalates can enter the body through several routes, including consumption of food or water, skin contact, and inhalation[7].
To understand the risks that phthalates might pose to people, it is essential first to assess the level of exposure to these chemicals in the population. Generally, researchers estimate phthalate exposure indirectly by measuring the concentration of phthalate breakdown products (metabolites) in biological samples, mostly urine. Thus, the U.S. National Center for Environmental Health (NCEH) at the Centers for Disease Control and Prevention (CDC) monitors phthalate exposure in the U.S. population using urine samples from people aged six and above who participate in the National Health and Nutrition Examination Survey (NHANES).
NHANES data collected between 2013 and 2014 showed detectable levels of 12 different phthalate metabolites in 40% to 99.5% (depending on the metabolite) of the 2,685 participants tested. A 2004 NCEH study using NHANES data from 1999 and 2000 showed similar results, with detectable levels of eight major phthalates in 89% to 98% of the population[8]. This study also found that phthalate levels in children and women are generally higher compared to those in adults and men, respectively.
These data demonstrated that exposure to phthalates is widespread across the U.S. population, and research has shown the same in Europe as well[9].
Phthalates impair fertility in rodents
Over three decades of research show that phthalates cause reproductive and developmental problems in rodents, particularly in males.
In 1977, Gray et al. published one of the first studies reporting such effects, showing that male rats fed with DEHP had smaller testicles and lower sperm production compared to unexposed males[10].
Later studies found that phthalate exposure during fetal development caused even more pronounced defects. Thus, feeding pregnant rodents with DEHP, MBP, DBP, BBP, and DiNP disrupted testosterone production in their male offspring, causing problems in testicle descent and sperm production, infertility, and malformations in the genito-urinary system[11-16]. This set of defects has been dubbed the “phthalate syndrome”[17].
While most of the effects of phthalates have been shown in males, problems in hormone production and fertility have also been reported in females[18]. For example, a 1987 study by Lamb et al. showed that exposing either male or female rats to DEHP before and during mating resulted in a lower number of live pups per litter compared to unexposed pairs[19]. DBP had a similar effect, but only in females. In contrast, DEP showed no effect on reproduction in either males or females.
In 1994, another study by researchers at North Carolina State University found that female rats fed with DEHP developed polycystic ovaries and problems ovulating[20].
Importantly, the effects of phthalates appear to be cumulative because exposing the animals to a mixture of several different phthalates or phthalates and other endocrine disruptors leads to more pronounced defects[21,22] that may impact several generations[23].
Results from animal studies don’t automatically translate to humans
It might seem reasonable to assume that a substance that causes reproductive problems in animals will have a similar effect in humans. However, this isn’t necessarily the case. While the effects on animals can be a warning signal, there are substantial differences between animal experiments and human exposure.
First, rodents’ biology differs from that of humans. This is one of the reasons why the results obtained in animals often don’t translate to people, as Ri Scarborough, a veterinarian and manager of the Cancer Research Program at the University of Melbourne, explained in an article for The Conversation. Scarborough illustrated these differences by citing the example of chocolate, a completely safe food for people that is nevertheless toxic for dogs. The reason is that, in contrast to humans, dogs lack the necessary enzymes to break down chocolate’s caffeine and theobromine, which accumulate in their bodies, causing toxicity.
In this regard, researchers at the Paris Diderot University analyzed the effects of several endocrine disruptors, including phthalates, on the formation of male hormones and sperm in rat, mouse, and human testes cultures[24]. They found that the response to the chemicals in rodent and human cultures was similar only in one-third of the cases, cautioning against extrapolating the results from rodents to humans.
Second, animal studies might not be representative of human exposure because many of the effects observed in rodents occur at much higher phthalate concentrations than those typical of human exposure[25]. The routes of exposure to some phthalates also differ between animals and humans because rodents generally receive the phthalates orally with food or water. While ingestion is also a main route of exposure to certain phthalates in people, others enter the body primarily through skin contact or inhalation.
These differences prevent us from drawing firm conclusions about the risks of phthalates to human health based only on animal studies. In order to more reliably establish the effects of phthalates on humans, we need human studies.
Several studies in humans observe a correlation between phthalates and reproductive issues, but this alone doesn’t establish causation
Evidence from clinical and epidemiological studies suggests a possible link between exposure to many endocrine disruptors, including phthalates, and reproductive, developmental, and metabolic problems in both men and women[26].
However, correlation alone doesn’t equal causation. That is, the fact that two variables change in relation to each other doesn’t necessarily mean that one caused the other. In the case of phthalates, there is no conclusive evidence showing that phthalates caused the problems described above.
We need to keep in mind that humans are exposed to thousands of chemicals every day, including heavy metals, herbicides, insecticides, and others. According to the U.S. Endocrine Society, people come into contact with more than 85,000 synthetic chemicals every day, and over 1,000 of them have the potential to interfere with hormone signaling based on their chemical properties. The U.S. CDC’s National Biomonitoring Program currently monitors the levels of more than 400 environmental chemicals in human samples. Even so, many of them aren’t well studied, so their effects on human health are unclear.
With people being exposed to so many chemicals, disentangling the individual effects of each compound becomes extremely challenging. Furthermore, there are other important factors besides environmental chemicals—including genetics, lifestyle factors, and underlying chronic conditions—that also impact fertility and need to be taken into account[27-29].
In recent decades, research has shown that environmental exposure to phthalates during fetal development correlates with problems in testosterone, sperm production, and testis development[20-32]. Exposure during fetal development and early childhood is also associated with an increased risk of developing certain chronic conditions later in life[33], including metabolic[34] and neurological[35-37] problems in both men and women.
Although the potential effects of phthalates on women’s fertility are less studied than in men, researchers have found that high phthalate levels correlate with poorer pregnancy rates and outcomes[38-42], and with pregnancy complications like gestational diabetes[43,44].
However, the results are inconsistent across different studies, and many studies found no correlations between phthalate exposure and metabolic[45], cognitive[46], and reproductive issues, even at high exposure levels.
For example, a research group in Sweden published a study in 2002 that looked at whether men exposed to high levels of DEHP in factories had more difficulties conceiving compared to unexposed men[47]. The authors analyzed 397 pregnancies from 193 men and found no differences in the time to conception between exposed and unexposed men.
In 2004, another study by researchers at George Washington University evaluated the long-term effects of DEHP exposure in 19 adolescents who required extracorporeal membrane oxygenation at birth[48]. This is a type of artificial life support in which a PVC machine helps the infants breathe and pump blood, exposing them to DEHP levels close to those causing reproductive problems in rodents[49]. All the adolescents showed growth, hormone levels, and gonadal function within the normal range for their sex and age.
In other words, the question of whether phthalates are causing reproductive problems in people, and if so, at what concentrations is extremely difficult to answer. While more research is warranted to better understand the impact of environmental chemicals on fertility, it is important not to lose sight of the many other possible contributing factors.
Are phthalates decreasing men’s penis size?
The claim that exposure to phthalates is “shrinking penises” went viral in 2021, following the publication of the book “Count Down” by Shanna Swan, an environmental epidemiologist at the Icahn School of Medicine at Mount Sinai.
Based on her research on endocrine disruptors for over two decades, Swan suggested that exposure to environmental pollutants is causing a decline in sperm counts and a reduction in penis size over time. In an interview for The Guardian in March 2021, Swan further speculated that “by 2045 we will have a median sperm count of zero”, meaning that most couples wanting to conceive would need to rely on assisted reproduction.
Swan’s statements fuelled headlines blaming environmental pollutants, including phthalates, for a collapse in fertility and “shrinking penises”. However, several experts consider the available evidence to be insufficiently strong to support these sensational claims.
Swan’s theory about a fertility collapse is mostly based on a review of global sperm count trends published by Levine et al. in 2017, in which Swan is the senior author[50]. By analyzing data from 185 studies and over 42,000 men, the authors reported a sperm count drop of 59.3% between 1973 and 2011, which they interpreted as evidence of a decline in fertility that they expect will continue in the following decades.
But other researchers questioned these conclusions, arguing that the declines reported by Levine et al. are still within the range that the World Health Organization considers “normal” and their impact on fertility is unclear. Allan Pacey, an andrologist at the University of Sheffield, explained to The New York Times that above a minimum threshold, a higher sperm count is unlikely to increase the chances of conception. This means that the assumption by Levine et al. that any decline in sperm counts must necessarily reduce male fertility isn’t well supported.
Tim Moss, associate professor of obstetrics and gynecology at Monash University in Melbourne, mentioned other caveats in an article for The Conversation. He also explained that while several studies have suggested a decline in sperm counts over recent decades[51,52], the causes for this apparent decline and its impact on fertility remain to be determined[53].
Moss also noted that the evidence suggesting that endocrine disruptors affect people’s fertility is reasonable but inconclusive[54]. Furthermore, many other factors, such as diet, obesity, and chronic disease, have a significant effect on fertility[53].
In fact, whether global sperm counts are actually declining is still under debate. Different studies have shown contradictory results, with some even reporting increased sperm counts in certain regions[55,56].
The claim that phthalates are causing a decrease in penis size is also not supported by strong evidence. The basis for this claim are two studies published by Swan et al. in 2005 and 2015 evaluating the effects of fetal phthalate exposure on the reproductive function of U.S. infants and toddlers[32,57].
Swan and colleagues measured the levels of different phthalate metabolites (DEHP, DEP, DBP, DBzP, and DiBP) in the urine of pregnant women and found that these levels correlated with incomplete testicular descent, reduced distance from the anus to the genitals (anogenital distance), and smaller penile width in their male descendants.
The anogenital distance is used as a marker of reproductive toxicity in rodents because shorter distances in males are associated with reduced testosterone levels, lower sperm counts, and reduced penis size, all symptoms of the phthalate syndrome.
However, the correlation between this metric and phthalate exposure isn’t well established in humans due to the limited number of studies and the inconsistencies in their results. While some studies have reported associations between high phthalate metabolite levels and reduced male anogenital distance and penis size[58,59], others found no significant associations[60,61]. Interpreting the relevance of the changes is also challenging due to the variability in the methodology and the lack of age-specific reference ranges for each sex until very recently[62].
In an email to Health Feedback, Linda Birnbaum, a former director of the National Institute for Environmental Health Sciences (NIEHS) as well as the National Toxicology Program (NTP), explained that talking about “‘shrinking penises’ is a little extreme”. However, she said, “there is evidence that phthalates cause male feminization in humans”.
Data on the evolution of penile size over time are scarce. One of the few studies addressing this question was published in the World Journal of Men’s Health in 2023[63]. This study evaluated changes in penile size—measured as penile flaccid, stretched, and erect length—between 1942 and 2021. To do that, the authors compared data from 75 studies involving more than 55,000 men. They found that, although penile length varied depending on the geographical region, the global erect length increased 24% between 1992 and 2021 across all age groups. The study didn’t identify trends in the other measurements.
In his article, Moss concluded, “while environmental pollution is a pressing concern, the evidence suggests the catastrophic collapse of human reproduction and accompanying penis shrinkage is thankfully a pretty unlikely prospect”.
Can we avoid exposure to phthalates?
Phthalates are so pervasive in our everyday lives that completely avoiding them is virtually impossible. Still, the Columbia University Mailman School of Public Health offers several steps people can take to reduce their exposure[64]. The most obvious one is to opt for PVC-free products, fragrance-free cosmetics and personal care products, and to avoid scented air fresheners.
Another perhaps less obvious way is to eat fresh foods when possible. Studies show that takeout food is associated with higher phthalate exposure[65,66], and that substituting processed and packaged food with fresh foods cuts DEHP metabolite levels by half[67].
But reducing population-level phthalate exposure will take more than just actions by individuals—regulatory action is also needed. In this regard, the U.S. has prohibited toys and childcare products containing more than 0.1% of eight types of phthalates, including DHEP, DBP, and BBP. The European Union and Canada have similar regulations, but they have also gone one step further, prohibiting the use of certain phthalates in cosmetic products that are allowed in the U.S.
In the last few years, many manufacturers have started to voluntarily replace phthalates in their products with alternative compounds. However, these new chemicals can also migrate out of the products and might pose similar health risks as the original compounds.
Conclusion
Chemicals with endocrine-disrupting potential are numerous and ubiquitous in modern life. Current evidence suggests that many of them might interfere with reproduction in both men and women, turning chemical exposure into a pressing public health concern.
But understanding the effects of phthalates on human health is complicated by several issues. Firstly, while animal studies have reported reproductive issues related to phthalate exposure, animals and humans differ in their biology, so these results may not necessarily translate to humans.
Secondly, while some studies in humans observed a correlation between phthalate exposure and reproductive issues, other studies didn’t. These studies also couldn’t rule out the possibility that the observed effects may have been due to other factors that affect fertility, such as genetics and other environmental toxins.
Thirdly, it’s still uncertain how changes in sperm count and penis size directly predict changes in human fertility. Therefore, alarming claims attributing a future reproductive catastrophe to environmental chemicals in general, and to phthalates in particular, aren’t adequately substantiated by the available evidence.
What is certain is that the effect of phthalates on human fertility needs further investigation. Until more data becomes available, practicing the precautionary principle—taking steps to reduce harm despite continuing scientific uncertainty—may be the safer approach going forward.
REFERENCES
- 1 – La Merrill et al. (2020) Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nature Reviews Endocrinology.
- 2 – Kelley et al. (2012) Identification of Phthalates in Medications and Dietary Supplement Formulations in the United States and Canada. Environmental Health Perspectives.
- 3 – Da Costa et al. (2023) Occurrence of phthalates in different food matrices: A systematic review of the main sources of contamination and potential risks. Comprehensive Reviews in Food Science and Food Safety.
- 4 – Schecter et al. (2013) Phthalate Concentrations and Dietary Exposure from Food Purchased in New York State. Environmental Health Perspectives.
- 5 – Colacino et al. (2010) Dietary Intake Is Associated with Phthalate Body Burden in a Nationally Representative Sample. Environmental Health Perspectives.
- 6 – Rudel et al. (2003) Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environmental Science Technology.
- 7 – Eales et al. (2022) Human health impacts of exposure to phthalate plasticizers: An overview of reviews. Environmental International.
- 8 – Silva et al. (2004) Urinary Levels of Seven Phthalate Metabolites in the U.S. Population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental Health Perspectives.
- 9 – Vogel et al. (2023) Current exposure to phthalates and DINCH in European children and adolescents – Results from the HBM4EU Aligned Studies 2014 to 2021. International Journal of Hygiene and Environmental Health.
- 10 – Gray et al. (1977) Short-term toxicity study of di-(2-ethylhexyl) phthalate in rats. Food and Cosmetics Toxicology.
- 11 – Wilson et al. (2008) Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. International Journal of Andrology.
- 12 – Shono et al. (2007) Time-specific effects of mono-n-butyl phthalate on the transabdominal descent of the testis in rat fetuses. BJU International.
- 13 – Mylchreest et al. (2000) Dose-Dependent Alterations in Androgen-Regulated Male Reproductive Development in Rats Exposed to Di(n-butyl) Phthalate during Late Gestation. Toxicological Sciences.
- 14 – Parks et al. (2000) The Plasticizer Diethylhexyl Phthalate Induces Malformations by Decreasing Fetal Testosterone Synthesis during Sexual Differentiation in the Male Rat. Toxicological Sciences.
- 15 – Gray et al. (2000) Perinatal Exposure to the Phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, Alters Sexual Differentiation of the Male Rat. Toxicological Sciences.
- 16 – Borch et al. (2005) Early testicular effects in rats perinatally exposed to DEHP in combination with DEHA—apoptosis assessment and immunohistochemical studies. Reproductive Toxicology.
- 17 – Foster (2006) Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology.
- 18 – Pocar et al. (2012) Exposure to Di(2-ethyl-hexyl) phthalate (DEHP) in Utero and during Lactation Causes Long-Term Pituitary-Gonadal Axis Disruption in Male and Female Mouse Offspring. Endocrinology.
- 19 – Lamb et al. (1987) Reproductive effects of four phthalic acid esters in the mouse. Toxicology and Applied Pharmacology.
- 20 – Davis et al. (1994) Di-(2-ethylhexyl) Phthalate Suppresses Estradiol and Ovulation in Cycling Rats. Toxicology and Applied Pharmacology.
- 21 – Howdeshell et al. (2008) Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environmental Research.
- 22 – Hannas et al. (2011) Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicological Sciences.
- 23 – Li et al. (2020) Prenatal exposure to a phthalate mixture leads to multigenerational and transgenerational effects on uterine morphology and function in mice. Reproductive Toxicology.
- 24 – Habert et al. (2014) Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction.
- 25 – Grandjean and Toppari. (2006) Possible effects of phthalate exposure in doses relevant for humans. International Journal of Andrology.
- 26 – Diamanti-Kandarakis et al. (2009) Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocrine Reviews.
- 27 – Mínguez-Alarcón et al. (2018) Caffeine, alcohol, smoking, and reproductive outcomes among couples undergoing assisted reproductive technology treatments. Fertility and Sterility.
- 28 – Winter et al. (2023) Can Dietary Patterns Impact Fertility Outcomes? A Systematic Review and Meta-Analysis. Nutrients.
- 29 – Ameratunga et al. (2023) Obesity and male infertility. Best Practice and Research Clinical Obstetrics and Gynaecology.
- 30 – Duty et al. (2003) Phthalate Exposure and Human Semen Parameters. Epidemiology.
- 31 – Duty et al. (2003) The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environmental Health Perspectives.
- 32 – Swan et al. (2005) Decrease in Anogenital Distance among Male Infants with Prenatal Phthalate Exposure. Environmental Health Perspectives.
- 33 – Bornehag et al. (2004) The Association between Asthma and Allergic Symptoms in Children and Phthalates in House Dust: A Nested Case–Control Study. Environmental Health Perspectives.
- 34 – Xia et al. (2018) Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence. Environment International.
- 35 – Shoaff et al. (2020) Association of Exposure to Endocrine-Disrupting Chemicals During Adolescence With Attention-Deficit/Hyperactivity Disorder–Related Behaviors. JAMA.
- 36 – Lee et al. (2018) Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environmental Research.
- 37 – Kim et al. (2011) Prenatal Exposure to Phthalates and Infant Development at 6 Months: Prospective Mothers and Children’s Environmental Health (MOCEH) Study. Environmental Health Perspective.
- 38 – Latini et al. (2003) In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environmental Health Perspectives.
- 39 – Mu et al. (2015) Levels of Phthalate Metabolites in Urine of Pregnant Women and Risk of Clinical Pregnancy Loss. Environmental Science Technology.
- 40 – Hauser et al. (2016) Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing in Vitro Fertilization: Results from the EARTH Study. Environmental Health Perspectives.
- 41 – Al-Saleh et al. (2019) Couples exposure to phthalates and its influence on in vitro fertilization outcomes. Chemosphere.
- 42 – Zhang et al. (2020) Association of Parental Preconception Exposure to Phthalates and Phthalate Substitutes With Preterm Birth. JAMA.
- 43 – James-Todd et al. (2016) Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environment International.
- 44 – Villanger et al. (2020) Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environment International.
- 45 – Maresca et al. (2016) Prenatal Exposure to Phthalates and Childhood Body Size in an Urban Cohort. Environmental Health Perspectives.
- 46 – Gascon et al. (2015) Prenatal exposure to phthalates and neuropsychological development during childhood. International Journal of Hygiene and Environmental Health.
- 47 – Modigh et al. (2022) Time to pregnancy among partners of men exposed to di(2-ethylhexyl)phthalate. Scandinavian Journal of Work, Environment and Health.
- 48 – Rais-Bahrami et al. (2004) Follow-Up Study of Adolescents Exposed to Di(2-Ethylhexyl) Phthalate (DEHP) as Neonates on Extracorporeal Membrane Oxygenation (ECMO) Support. Environmental Health Perspectives.
- 49 – Tickner et al. (2001) Health risks posed by use of Di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: A critical review. American Journal of Industrial Medicine.
- 50 – Levine et al. (2017) Temporal trends in sperm count: a systematic review and meta-regression analysis. Human Reproduction Update.
- 51 – Lokeshwar et al. (2020) Decline in Serum Testosterone Levels Among Adolescent and Young Adult Men in the USA. European Urology.
- 52 – Cheng et al. (2018) Testicular cancer. Nature Reviews Disease Primers.
- 53 – Mann et al. (2020) Reasons for worldwide decline in male fertility. Current Opinion in Urology.
- 54 – Green et al. (2021) Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environmental Research.
- 55 – Becker and Berhane. (1997) A meta-analysis of 61 sperm count studies revisited. Fertility and Sterility.
- 56 – Auger et al. (2022) Spatiotemporal trends in human semen quality. Nature Reviews Urology.
- 57 – Swan et al. (2015) First trimester phthalate exposure and anogenital distance in newborns. Human Reproduction.
- 58 – Bornehag et al. (2015) Prenatal Phthalate Exposures and Anogenital Distance in Swedish Boys. Environmental Health Perspectives.
- 59 –Suzuki et al. (2012) Foetal exposure to phthalate esters and anogenital distance in male newborns. International Journal of Andrology
- 60 – Huang et al. (2009) Association between prenatal exposure to phthalates and the health of newborns. Environment International.
- 61 – Jensen et al. (2016) Prenatal Exposure to Phthalates and Anogenital Distance in Male Infants from a Low-Exposed Danish Cohort (2010-2012). Environmental Health Perspectives.
- 62 – Fischer et al. (2020) Anogenital Distance in Healthy Infants: Method-, Age- and Sex-related Reference Ranges. The Journal of Clinical Endocrinology and Metabolism.
- 63 – Belladelli et al. (2023) Worldwide Temporal Trends in Penile Length: A Systematic Review and Meta-Analysis. The World Journal of Men’s Health.
- 64 – Martin et al. (2022) Lifestyle interventions to reduce endocrine-disrupting phthalate and phenol exposures among reproductive age men and women: A review and future steps. Environment International.
- 65 – Varshavsky et al. (2018) Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environment International.
- 66 – Zota et al. (2016) Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environmental Health Perspectives.
- 67 – Rudel et al. (2011) Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environmental Health Perspectives.
